Fusion protein TpG, encoding gene thereof, recombinant plasmid, strain and application
A fusion protein and recombinant plasmid technology, applied in the field of protein genetic engineering, can solve the problems of limited biomedical applications, short half-life, lack of structural domains, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
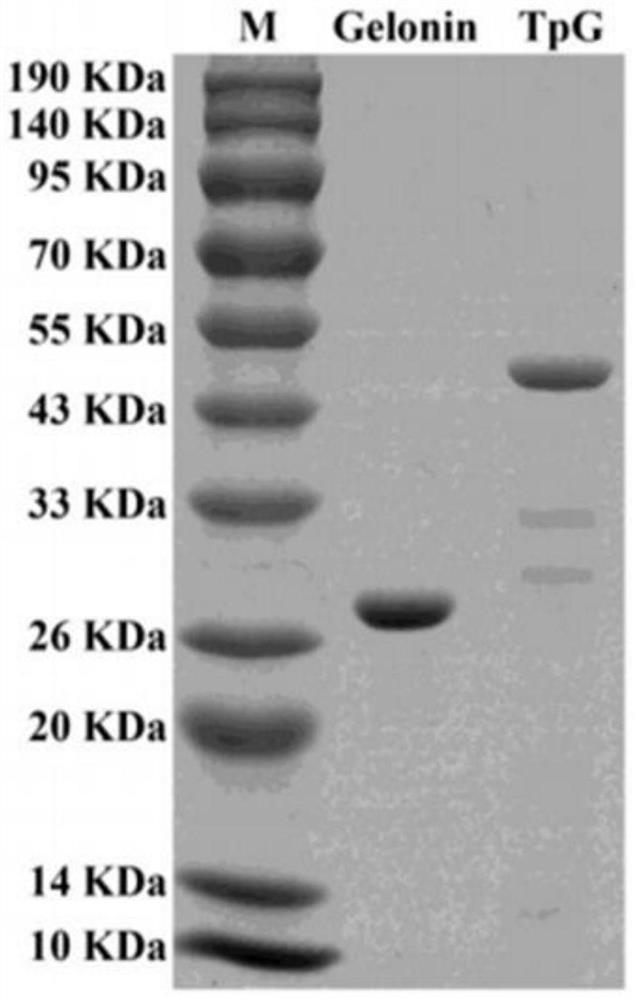
[0025] Preparation and characterization of embodiment 1, TpG
[0026] 1. Preparation of TpG
[0027] 1.1 Synthesis of pHLIP-Gelonin
[0028] The nucleic acid sequence of pHLIP-Gelonin (as shown in the 478th to 1344th positions of SEQ ID NO.2) was obtained through literature review, and then the gene pHLIP-Gelonin was synthesized by Shanghai Sangon Biological Co., Ltd. and ligated in pUC57 On the empty plasmid, the bacterial solution containing the pUC57-pHLIP-Gelonin recombinant plasmid was obtained.
[0029] 1.2 Construction of plasmids
[0030] (1) Extraction of pUC57-pHLIP-Gelonin and pET32a plasmids: Inoculate the pUC57-pHLIP-Gelonin and pET32a strains in 5 mL LB medium containing Amp (100 ng / μL) respectively, and culture in a constant temperature shaker at 37°C for 12- After 16 hours, the cells were collected by centrifugation at 3500 rpm for 5 minutes. The pET32a and pUC57-pHLIP-Gelonin plasmids were extracted by the SanPrep column plasmid DNA mini-extraction kit, an...
Embodiment 2
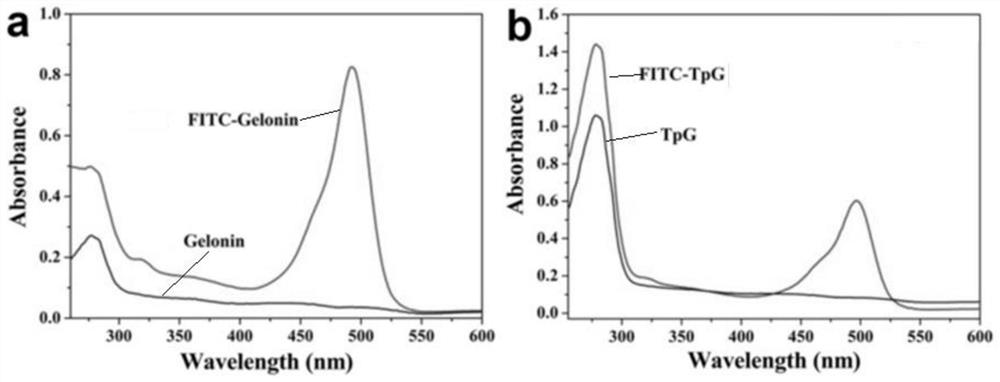
[0067] Embodiment 2, the in vitro antitumor activity evaluation of TpG
[0068] 1. Cell culture
[0069] Both SKOV3 cells and HCT-8 cells were cultured using RPMI 1640 medium, which was composed of serum-free RPMI 1640 medium, fetal bovine serum (FBS) and antibiotics (penicillin / streptomycin) at 89:10: 1 ratio for configuration. A549 cells were cultured in DMEM medium containing 10% FBS and 1% antibiotics (penicillin / streptomycin). All cells were kept at 37°C with 5% CO 2 cultured in a constant temperature cell incubator. The fresh medium was replaced in time according to the growth of the cells. When the cells grew to 80%, they were digested with 0.25% trypsin (without EDTA), and passed on to new cell culture flasks.
[0070] 2. FITC-labeled Gelonin and TpG
[0071] (1) Dissolve FITC-SCN in PBS buffer and prepare 1 mg / mL FITC labeling solution.
[0072] (2) Mix 1 mg of TpG (stored in PBS buffer) with FITC at a molar ratio of 1:2, make up to 1 mL with PBS buffer, and pla...
Embodiment 3
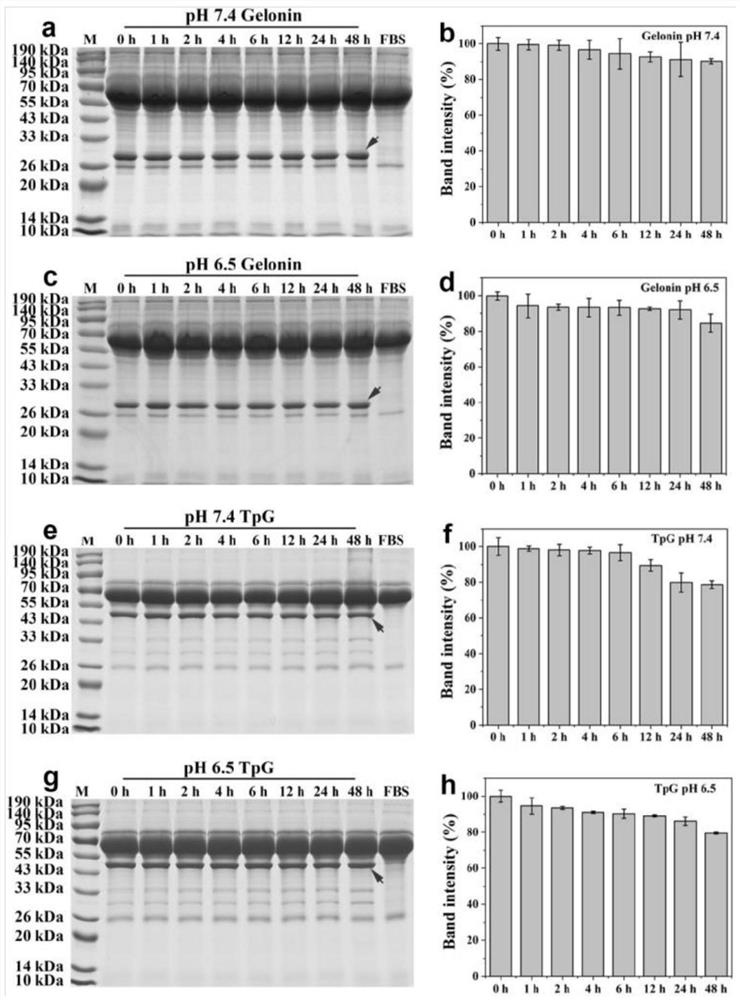
[0121] Embodiment 3, the in vivo antitumor activity evaluation of TpG
[0122] 1. Establishment of tumor-bearing nude mouse model
[0123] Female athymic BALB / c nude mice (4 weeks old) were purchased from the China Institute for Food and Drug Control, and were raised in the SPF animal room of the China Institute of Radiation Protection, and fed with sterile standard feed. The animal experiments were approved by the Experimental Animal Management and Use Ethics Committee of the Chinese Institute of Radiation Protection, and the approval number is CIRP-IACUC-(R)2020021. SKOV3 cells were suspended in saline, and each mouse was injected with 100 μL (containing 1×10 7 cells), the cell suspension was inoculated into the axilla of the right forelimb of nude mice by subcutaneous injection, and a xenograft model of human ovarian cancer SKOV3 nude mice was established.
[0124] 2. In vivo tumor inhibition experiment
[0125] The average tumor volume reaches 200mm 3 (defined as the 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com