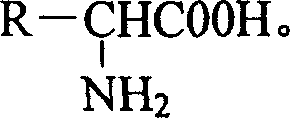Amino acid racemization method
An amino acid, racemization technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., to achieve the effects of fast reaction speed, high product yield, and complete racemization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: 20g of L-tyrosine, 100ml of anhydrous acetic acid and 22ml of acetic anhydride were added to a 250ml three-neck flask, stirred, and heated to 110°C in an oil bath. The initial time of heating was recorded and the system cleared after 10 minutes. Take 10ml sample, dilute it to 25ml with deionized water and measure its optical rotation value. After that, 10 ml of samples were taken every 10 minutes to measure the optical rotation value until it was 0. Tyrosine reaches 100% racemization, and the required racemization time is 30 minutes.
[0016] The obtained racemized solution was distilled off the solvent under reduced pressure to obtain a wine red viscous substance, which was added with 100ml of 3N HCl solution and refluxed for two hours to completely hydrolyze the acetyl group. Use sodium hydroxide solution to adjust the pH value of the solution to about 3, add activated carbon to decolorize, filter to remove activated carbon, continue to dropwise add sodiu...
Embodiment 2
[0017] Example 2: 20g of L-tyrosine, 100ml of anhydrous acetic acid and 22ml of acetic anhydride were added to a 250ml three-neck flask, stirred, and heated to 100°C in an oil bath. The initial time of heating was recorded and after 40 minutes the system was clear. Take 10ml sample, dilute it to 25ml with deionized water and measure its optical rotation value. After that, 10 ml of samples were taken every 10 minutes to measure the optical rotation value until it was 0. Tyrosine reaches 100% racemization, and the required racemization time is 60 minutes.
Embodiment 3
[0018] Example 3: 20 g of L-tyrosine, 100 ml of anhydrous acetic acid and 22 ml of acetic anhydride were added to a 250 ml three-neck flask, stirred, and heated to 80° C. in an oil bath. The initial time of heating was recorded and the system cleared after 80 minutes. Take 10ml sample, dilute it to 25ml with deionized water and measure its optical rotation value. Thereafter, 10 ml of samples were taken every 30 minutes to measure the optical rotation value until it was 0. Tyrosine reaches 100% racemization, and the required racemization time is 140min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com