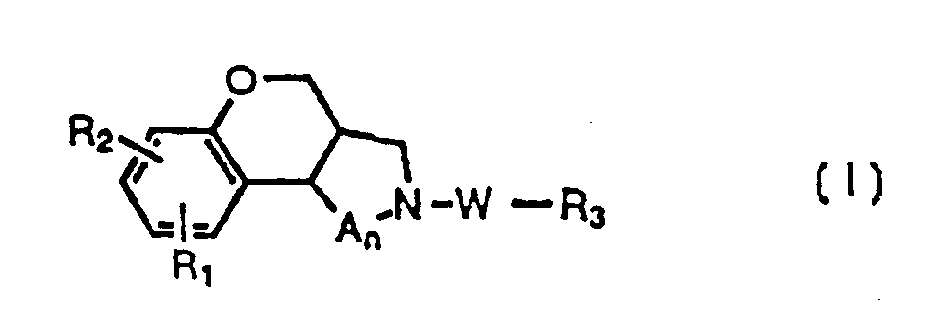
Benzopyranopyrrole and benzopyranopyridine alpha-1 adrenergic compounds
A compound, halogen technology, applied in the field of benzopyranopyrrole and benzopyranopyridine alpha-1 adrenergic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0342] Example 13-[4-((3aR, 9bR)-cis-9-methoxy-1.2.3.3a.4.9b-hexahydro-[1]-benzopyrano[3,4-c]pyrrole -2-yl)butyl]-pyrido[2',3':4,5]thieno[3,2-d]pyrimidine-
[0343] 2,4(1H,3H)-Diketone Hydrochloride
Embodiment 1A
[0344] Example 1A (3aR, 9bR)-cis-9-methoxy-2-(R)-α-methylbenzyl-1,2,3,3a,4,9b-hexahydro-
[0345] [1]-Benzopyrano[3,4-c]pyrrol-4-one
[0346] 5-Methoxycoumarin (22.3 g, 126 mmol) and trifluoroacetic acid (0.97 mL, 12.6 mmol) were dissolved in CH 2 Cl 2 (200mL) and cooled to 0°C. To the stirred solution was added N-methoxymethyl-N-trimethylsilylmethyl-(R)-α-methylbenzylamine (63.4 g, 252 mmol) over 30 minutes. The reaction was stirred at 0°C for an additional 30 minutes, then at 25°C for 1 hour. The reaction mixture was washed with 5% NaHCO 3 After washing, the organic layer was dried and evaporated. The resulting oil was suspended in ether, and after 2 hours, the title compound was collected by filtration (15.4 g, 38%). 1 HNMR (300MHz, CDCl 3 )δ1.36(d, 3H), 2.41(dd, 1H), 3.04(d, 1H), 3.05-3.15(m, 2H), 3.23(m, 1H), 3.32(1, 1H), 3.75(m , 1H), 3.79 (s, 3H), 6.61 (d, 1H), 6.67 (d, 1H), 7.18 (t, 1H), 7.20-7.35 (m, 5H).
Embodiment 1B
[0347] Example 1B (3aR, 9bR)-cis-9-methoxy-2-(R)-α-methylbenzyl-1,2,3,3a,4,9b-hexahydro-
[0348] [1]-Benzopyrano[3,4-c]pyrrole
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com