A pharmaceutical preparation comprising an angiotension II2 type receptor agonist, and use thereof
An angiotensin and receptor agonist technology, applied to pharmaceutical preparations containing angiotensin II type 2 receptor agonists and its application fields, can solve unstable blood pressure regulation, increase allergic performance and upper airway stimulation Hazardousness, accumulation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
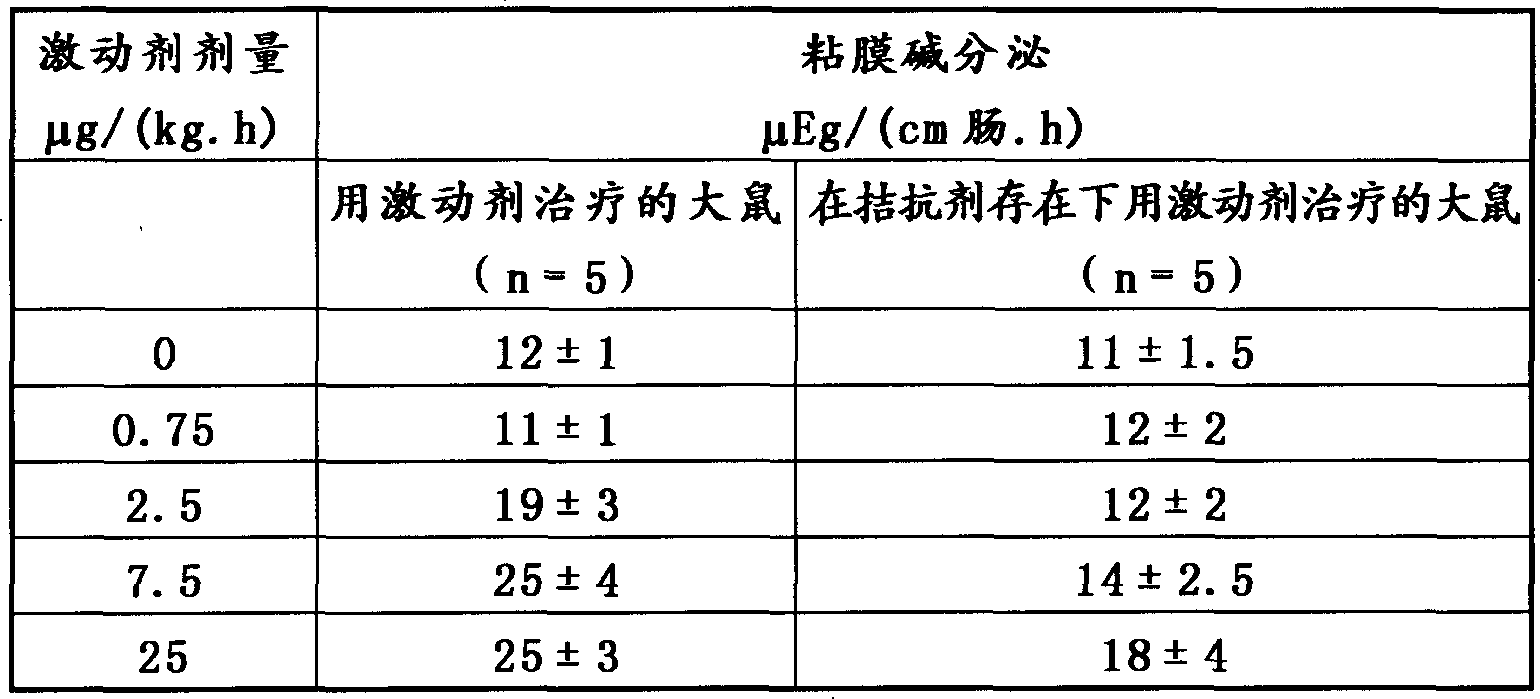
[0031] In this in vivo example, the peptide purchased from SIGMA, the angiotensin II type 2 receptor agonist p-aminophenylalanine 6 - Angiotensin II (Product No. A1811). The agonist was administered intravenously to rats anesthetized with chloralose, prepared for the in situ measurement of base secretion in the duodenal mucosa (Flemstrom et al. in Am. J. Physiol. ) 1982, 243: the method described in G348). As a result, it was found that increasing the infusion rate of the compound significantly stimulated mucosal base secretion in a dose-dependent manner, as shown in the table below.
[0032] To further exemplify the receptor specificity of this agonist, it was also administered in the presence of a specific angiotensin II type 2 receptor antagonist (PD123319, 200 μg / kg iv), with positive effects on systemic parameters such as mean Arterial blood pressure did not have any significant side effects. The alkali secretion of the mucous membrane is counteracted. This is also il...
Embodiment 2
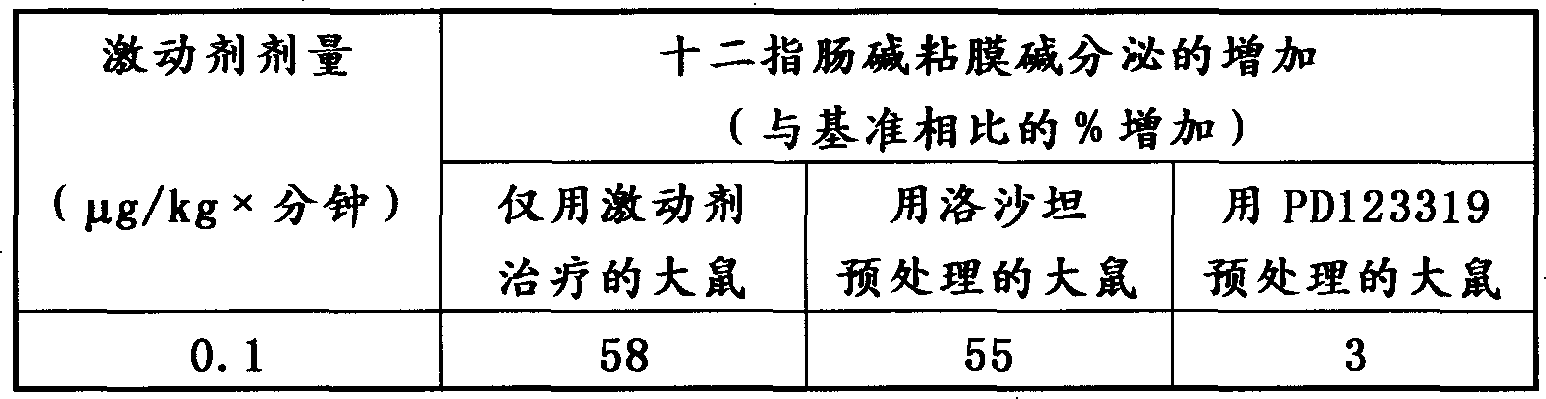
[0036] In this in vivo example, use is purchased from NEOSYSTEM S. A. , a peptide from France, the selective angiotensin II type 2 receptor agonist CGP 42112A (N-α-nicotinoyl-Tyr-(N-α-CZB-Arg)Lys-His-Pro-Ile-OH)( Product No. SC431). The agonist was administered intravenously as described in Example 1 to chloralose-anesthetized rats prepared for in situ measurement of base secretion in the duodenal mucosa.
[0037] As a result, it was found that, as shown in Table 2 below, a single dose of CGP 42112A (0.1 µg / kg x min) increased duodenal mucosal bicarbonate secretion. As shown in the table below, co-administration with the specific angiotensin II type 1 receptor antagonist losartan (10 mg / kg iv) had no effect on the stimulation response.
[0038] To further illustrate the receptor specificity of this agonist, CGP 42112A was also administered together with the specific angiotensin II type 2 receptor antagonist PD123319 (200 [mu]g / kg iv). The alkali secretion of the duodenal m...
Embodiment 3
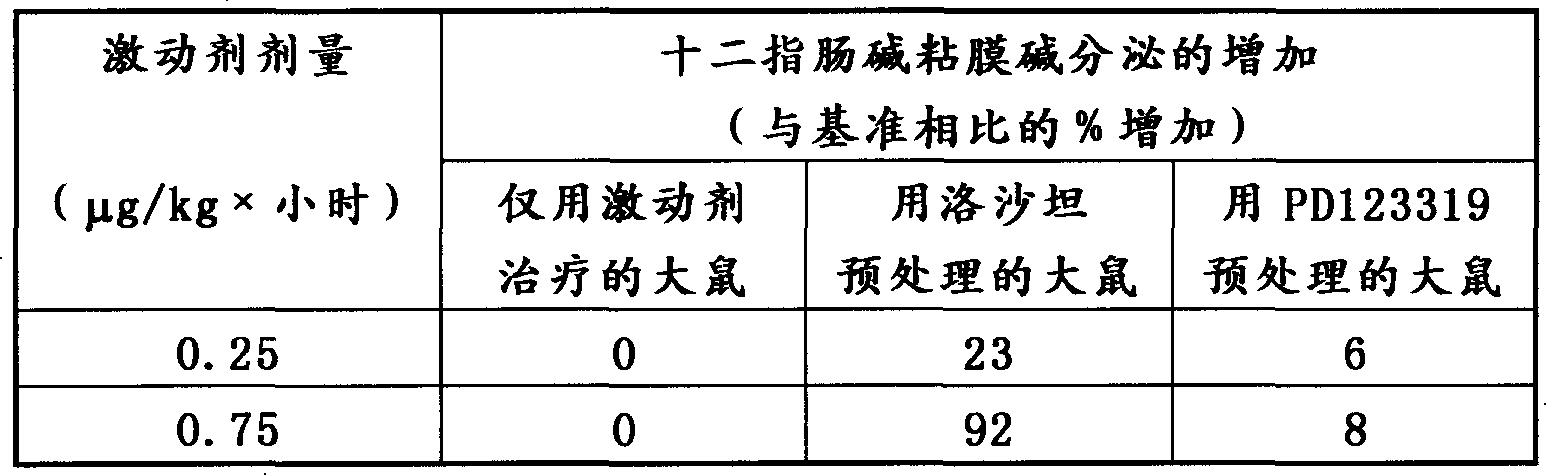
[0041] In this in vivo example, angiotensin II, the endogenous ligand for the angiotensin II receptor, purchased from SIGMA, was used. The agonist was administered intravenously as described in Example 1 to rats anesthetized with chloralose prepared for in situ assays.
[0042] As a result, it was found that, as shown in Table 3 below, the administration of angiotensin II alone could not increase the base secretion of the duodenal mucosa.
[0043] Following pretreatment with the specific angiotensin II type 1 receptor antagonist losartan (10 mg / kg iv), infusion of angiotensin II stimulated base secretion in the duodenal mucosa as described in the table below.
[0044] Co-administration with the specific angiotensin II type 2 receptor antagonist PD123319 (200 μg / kg intravenously) effectively counteracted this stimulatory response as described in the table below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com