Preparation method of optically active beta-hydroxy ketone
A photoactive, hydroxy ketone technology, applied in the field of preparing photoactive β-hydroxy ketone, can solve the problems of high preparation cost, low catalytic efficiency, complicated preparation procedures, etc., and achieves simple and easy to obtain raw materials, low preparation cost and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
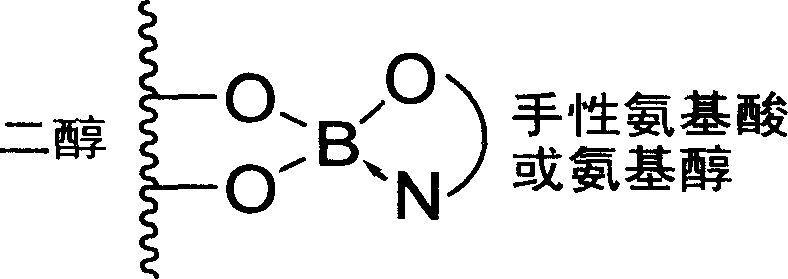
Method used
Image
Examples
Embodiment l
[0019] This example provides a representative procedure that will be used in the following examples: In a Schlenk test tube equipped with a stir bar and methyl ketone or monosubstituted methyl ketone or cyclic ketone, add There are 0.01-1.50 equivalents of chiral spiro borate catalyst, stirred at -25 to 80°C for 15 minutes, and then added aldehyde, the molar ratio of aldehyde and methyl ketone or monosubstituted methyl ketone or cyclic ketone is 1.00:1.00~10.0 , and continue to stir at this temperature for 10-100h. Add 0.1-10.0 equivalents of saturated NH 4 Cl aqueous solution or dilute hydrochloric acid or dilute sulfuric acid solution, stirred, extracted, combined organic phase, dried (anhydrous Na 2 SO 4 ), after concentration, the catalyst crystals were left to separate out, separated, and the mother liquor was subjected to flash column chromatography on silica gel to obtain photoactive β-hydroxy ketones.
[0020] Such as: the addition of acetone and benzaldehyde, the s...
Embodiment 2
[0022] According to the operating procedure of Example 1, excess acetone and benzaldehyde were added at 20°C with 0.3 equivalent catalyst, and extracted with ether to obtain (R)-4-hydroxyl-4-phenyl-2-butanone, [α ] D 25 =+43.02 (c=0.30, inCHCl3 ), 11% yield, 70% ee
Embodiment 3
[0024] According to the operating steps of Example 1, acetone and benzaldehyde were added in THF at -15°C with 0.3 equivalent catalysts to obtain (R)-4-hydroxyl-4-phenyl-2-butanone, [α] D 25 =+50.59(0.09, in CHCl 3 ), 64% yield, 83% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com