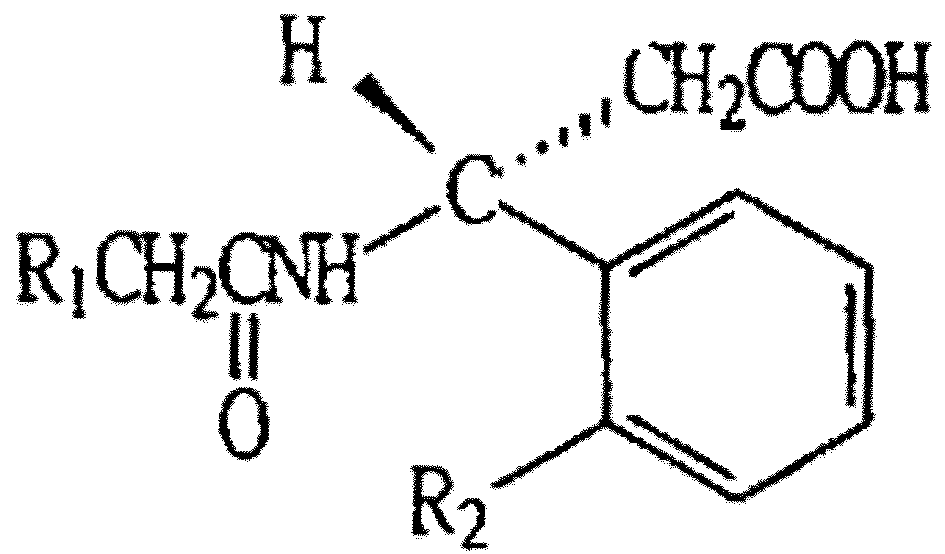
(R)-beta-phenylacetylamino-beta-phenylpropionic acid compounds
A technology of phenylacetamide and phenylpropionic acid, applied in the field of medicine, can solve problems such as hypoglycemia and lower glucose levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0006] (R)-β-Phenylacetamido-β-O-methoxyphenylpropionic acid (code name AD)
[0007] Add 20.9g of 3-amino-3-(o-methoxyphenyl)propionic acid to 100ml of chloroform and 100ml of ethanol, add 4.4g of sodium hydroxide, stir, add 22ml of triethylamine, and cool the solution to 0°C. 16.65g of phenylacetyl chloride was dissolved in 50ml of chloroform and added dropwise to the above solution. The rate of dropping was controlled so that the temperature of the inner bath was lower than 5°C. After the dropping, the reaction was kept warm for 60 minutes, raised to room temperature, filtered with suction, and the filtrate was concentrated to the original Add 1 / 3 of the volume, add 100ml of water to the residue, shake well, adjust the pH to 3 with hydrochloric acid, stand still, separate the water layer, adjust the pH to 3 again, extract with chloroform, combine the organic phases, and dry with anhydrous sodium sulfate. The desiccant was filtered off, the filtrate was concentrated, and the resi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com