i(in Vitro) induction of antigen-specific T-cells using dendritic cell-tumor cell or dendritic cell-viral cell derived immunogens
A technology of dendritic cells and tumor cells, applied in the direction of virus antigen components, cell culture active agents, fusion cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-D
[0054] Fusion of Example 1-DC and Tumor Cells
[0055] Dendritic cells were prepared from bone marrow as generally described in Celluzzi et al., J. Exp. Med. 183:283-287 (1996), in which GM-CSF was used in reference. Briefly, bone marrow cells were depleted of lymphocytes and cultured in 10% FCS containing RPMI 1640 from Irvine Scientific, Santa Ana, California at a cell density of 5×10 5 Cells / ml, using granulocyte-macrophage colony-stimulating factor (GM-CSF) from Sigma Chemical Company, St.Louis, Missouri, at a concentration of 10 3 U / ml. Loosely attached cells were collected for fusion on day 6. The concentrations of CD86 (B7.2) and MHC class II (I-A+) antigens expressed by DCs were 50-75% as measured by flow cytometry.
[0056] On day 6, DCs were fused with B16 or 3LL cells at a ratio of DCs to tumor cells of 6:1, using polyethylene glycol at 37°C. After washing by centrifugation, the fused cells were cultured overnight in RPMI1640 (10% FCS) at 37°C.
Embodiment 2
[0057] Example 2 - Preparation of co-culture of dendritic cells and tumor cells
[0058] Dendritic cells were prepared according to the method in Example 1. On day 6, dendritic cells were used to form co-cultures with B16 cells or 3LL cells. Various DCs and tumor cells were placed in test tubes to prepare various DC / tumor cell co-cultures. Centrifuge to form a cell pellet. Cell pellets were then diluted with RPMI (10% FCS) and incubated overnight at 37C in a 5% CO2 incubator. The ratio of DC:tumor cells is about 6:1. Co-cultured products were prepared to further assess whether tumor antigens were closely associated with DCs, and to assess whether soluble factors released from tumors were present on DCs.
Embodiment 3
[0059] Embodiment 3 - the effect of fusion and co-cultivation
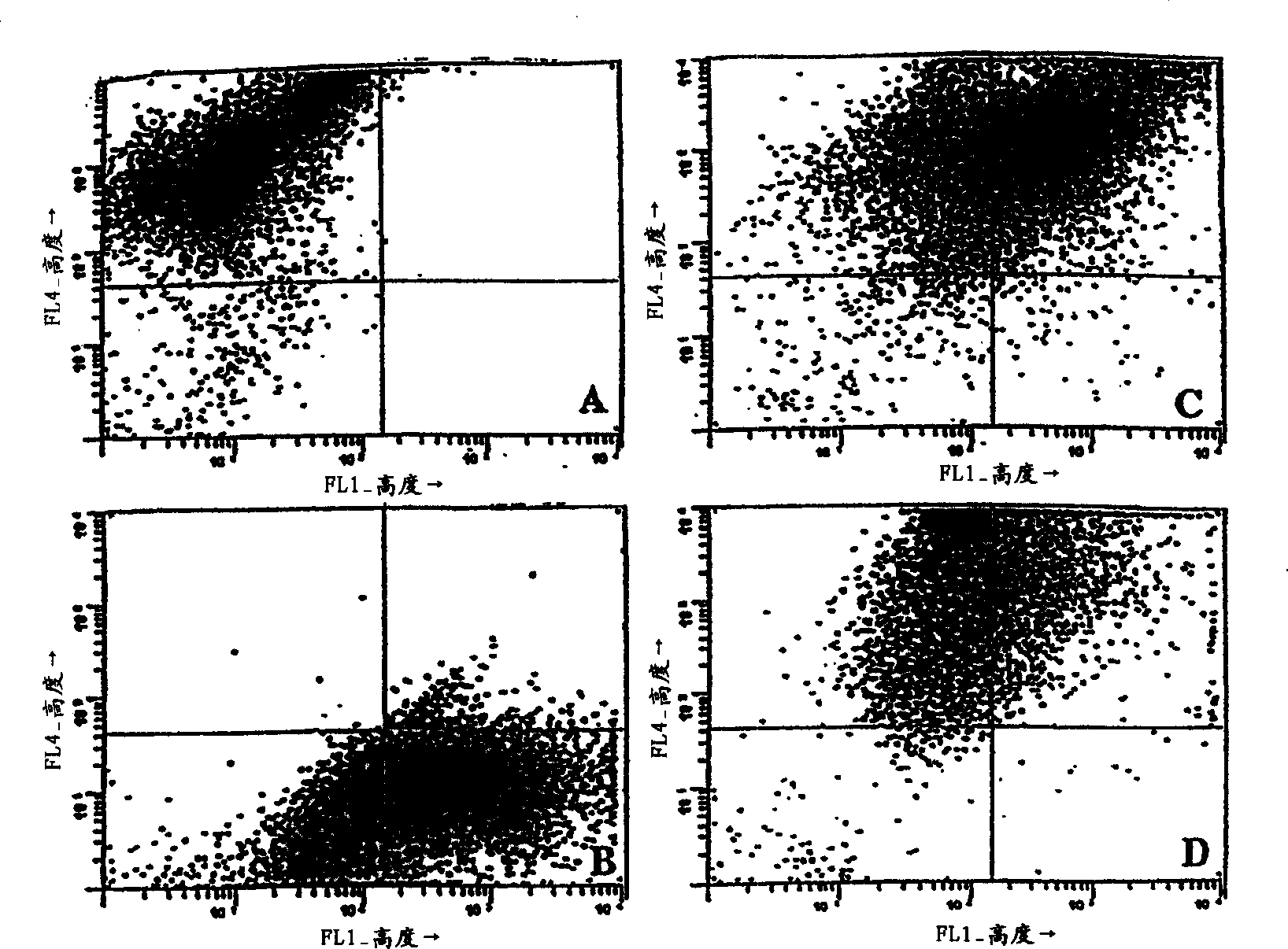
[0060] To determine the effect of fusion and co-culture, various cell types - DC, B16 and 3LL - were stained with different lipophilic fluorescent dyes prior to fusion and analyzed by flow cytometry. Tumor cells were stained with DiO and DCs were stained with DiI, both obtained from Molecular Probes, Inc., Eugene, Oregon. After extensive washing, cells were fused or co-cultured and incubated overnight at 37°C. The resulting cells were then fixed in 2% paraformaldehyde and then characterized by an Argon / HeNe duellaser on a Becton Dickinson Facstar Plus, available from Becton Dickinson Immunocytometry Systems, San Jose, CA.
[0061] For scatter characteristics of various cell types see figure 1 . Staining of individual cells showed two different patterns, with DC (DiI) shifting upwards compared to unstained controls (not shown) ( figure 1 Upper left quarter of A) or B16 tumor cells (DiO) moving to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com