Isolation of taxanes
一种紫杉烷类、化合物的技术,应用在有机化学等方向,能够解决成本高、增加成本、溶剂不能被容易地回收等问题,达到降低生产成本、降低开销的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
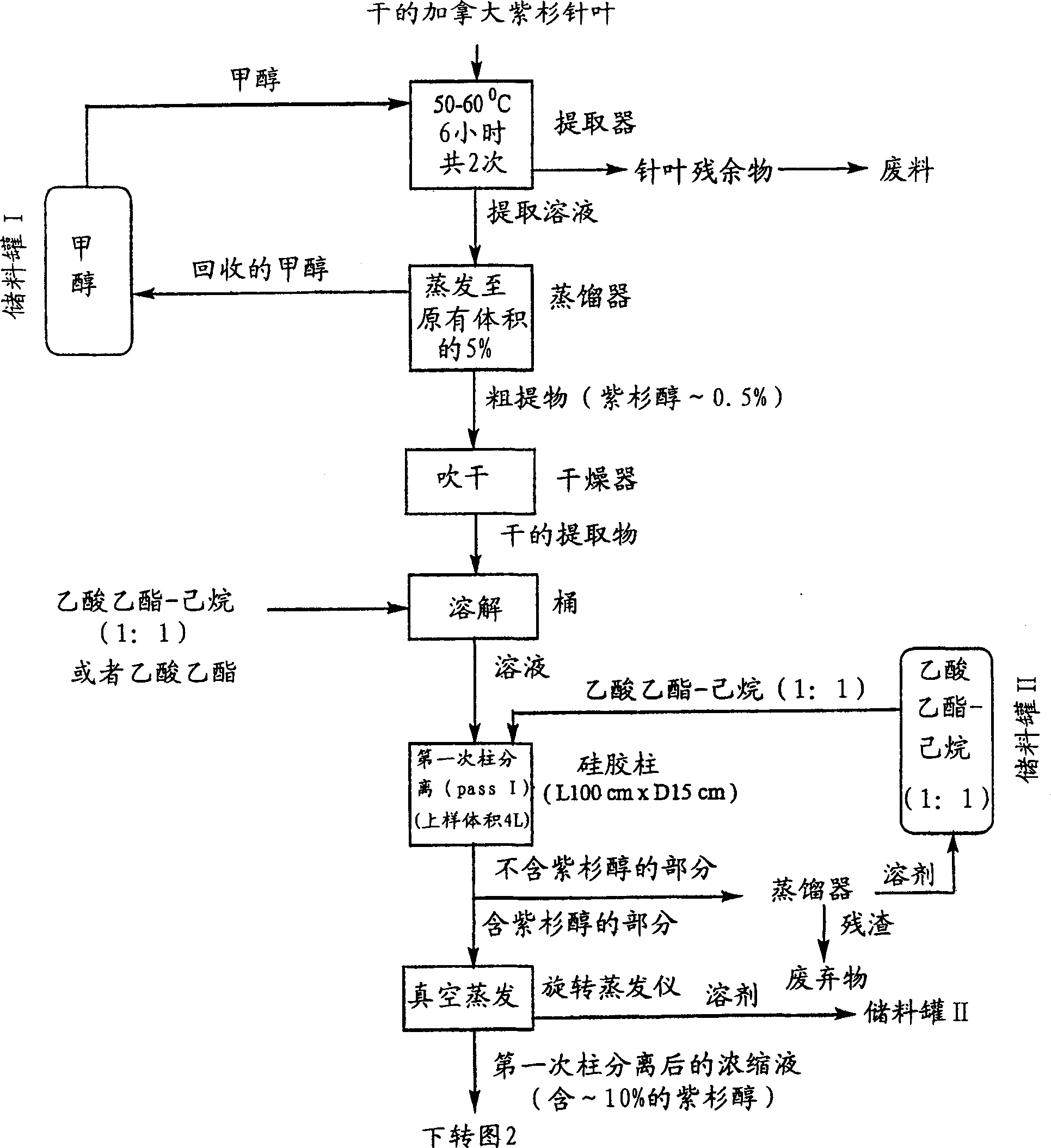
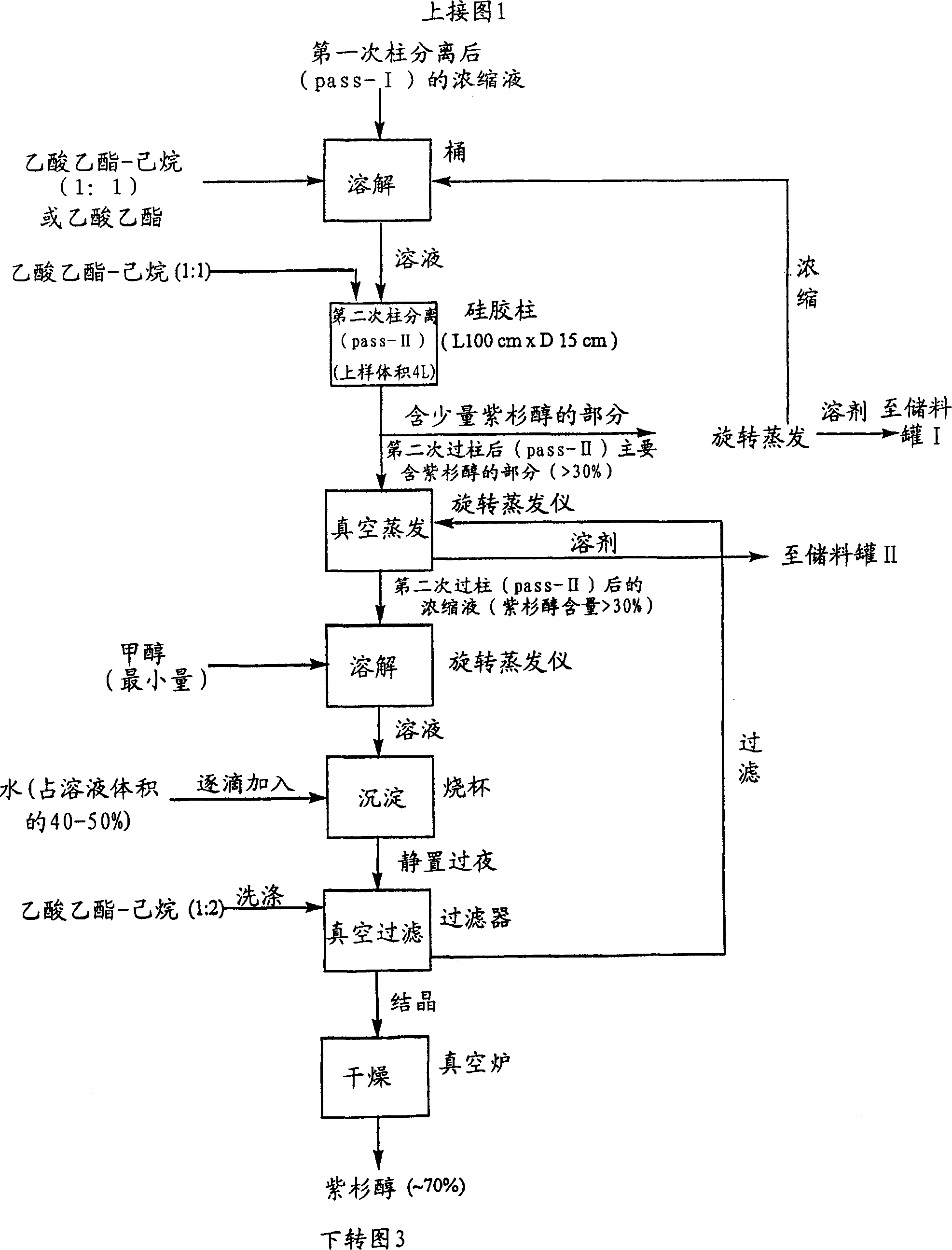
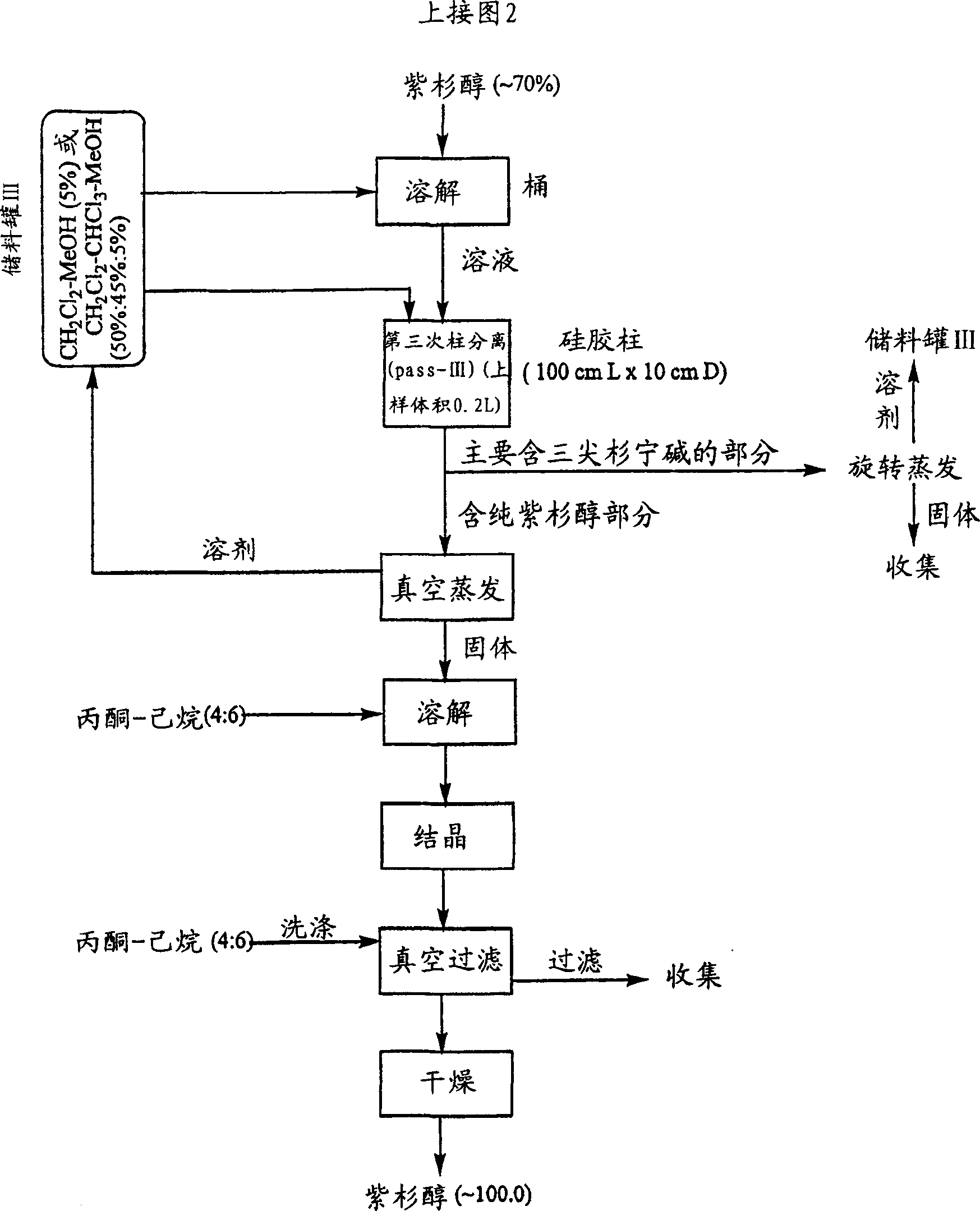
[0035] All chemicals used were commercially available and were tested analytically before use. All glass chromatographic columns and silica gel were purchased from China Far East Pharmaceutical Instrument Co., Ltd., and industrial solvents were purchased from Stanchen Company in Canada. The standard products used in HPLC, such as paclitaxel, cephalomannine, 9-dihydro-13- Acetyl-baccatin III was purchased from Hauser Company of the United States, fluorescent silica gel TLC thin plate was purchased from Waters Company of the United States, and a Dionex 500 HPLC system was used to analyze the components in the method of the present invention.
[0036] The crude extract (0.5 kg) derived from the needles of Taxus canadensis was dissolved in 4 L of ethyl acetate. The solution was loaded into an industrial-grade glass chromatographic column (15 cm×100 cm), which had previously been filled with silica gel powder (1×250 mesh) for about 70% of the column height. Elution was performed u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com