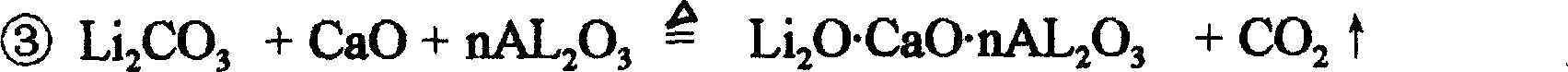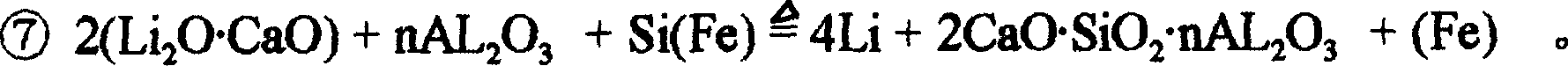Lithium preparing process with lithium carbonate
A lithium carbonate, purpose technology, applied in the field of metal smelting, can solve the problems of low product purity, large equipment investment and high product cost, and achieve the effects of high purity, less investment demand and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] ① According to lithium carbonate (Li 2 CO 3 ): quicklime (CaO): alumina (Al 2 o 3 ) = 0.8: 0.819: 0.256 by weight ratio and take powdery lithium carbonate with a purity greater than 99% and a particle size of less than 65 meshes, powdered quicklime with a purity greater than 96% and a particle size of less than 80 meshes and a powder with a purity greater than 98% and a particle size of less than 65 meshes Shaped alumina, and mix the three evenly;
[0027] ② Make the particle size n of the powdery mixture obtained in the previous step: n=10mm, and the specific gravity is 1.6g / cm 3 solid particles;
[0028] ③Use the rotary kiln to calcinate the solid particles obtained in the previous step at a temperature of 900°C until the lithium carbonate in the particles is completely decomposed, and the carbon dioxide contained in it is exhausted, and the calcined solid particles obtained are used for later use; the calcining process Must use pure heat source, can not use impu...
Embodiment 2
[0035] ① According to lithium carbonate (Li 2 CO 3 ): quicklime (CaO): alumina (Al 2 o 3 ) = 0.925: 0.8245: 0.258 by weighing the powdered lithium carbonate with a purity greater than 99% and a particle size less than 65 mesh, powdered quicklime with a purity greater than 96% and a particle size less than 80 mesh and powder with a purity greater than 98% and a particle size less than 65 mesh Shaped alumina, and mix the three evenly;
[0036] ② Make the particle size n of the powdery mixture obtained in the previous step: n=15mm, and the specific gravity is 1.8g / cm 3 solid particles;
[0037] ③Calcinate the solid particles obtained in the previous step at a temperature of 1000°C in a rotary kiln until the lithium carbonate in the particles is completely decomposed, and the carbon dioxide contained in it is exhausted, and the calcined solid particles obtained are used for later use; the calcining process Must use pure heat source, can not use impure heat source;
[0038] 4...
Embodiment 3
[0044] ① According to lithium carbonate (Li 2 CO 3 ): quicklime (CaO): alumina (Al 2 o 3 ) = 1.05: 0.83: 0.26 by weight ratio, respectively weighing the powdered lithium carbonate with a purity greater than 99% and a particle size of less than 65 meshes, powdered quicklime with a purity greater than 96% and a particle size of less than 80 meshes, and powders with a purity greater than 98% and a particle size of less than 65 meshes. Shaped alumina, and mix the three evenly;
[0045] ② Make the particle size n of the powdery mixture obtained in the previous step: n=20mm, and the specific gravity is 2g / cm 3 solid particles;
[0046] ③Calcinate the solid particles obtained in the previous step at a temperature of 1100°C in a rotary kiln until the lithium carbonate in the particles is completely decomposed, and the carbon dioxide contained in it is exhausted, and the calcined solid particles obtained are used for later use; the calcining process Must use pure heat source, can ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com