Educated NKT cells and their uses in the treatment of immune-related disorder
An NKT cell, immune-related technology, applied in the field of educated NKT cells and their use in the treatment of immune-related disorders, can solve the problem that the precise mechanism has not yet been discovered.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0245] Tolerance induction in experimental colitis
[0246] To assess the role of tolerance induction in the experimental colitis model, six groups of mice consisting of 20 animals each were studied (Table 1). All mice were rectally challenged with TNBS (groups A, B, D and E) or normal saline (control groups C and F) on day 1 of the study. From the day of colitis induction, mice of all groups were fed every other day for 11 days (50 μg / mouse). Groups B and E included mice fed with colitis-extracted protein (CEP). Mice in groups A, C, D and F were supplied with bovine serum albumin (BSA, 50 μg / mouse). All groups of mice were sacrificed 14 days after colitis induction. Mice in groups D-F were treated with anti-NK1.1 anti-mouse monoclonal antibody 36 hours before study termination as described above. Mice in groups A-C were not NK1.1-null.
[0247]Table 1
[0248] Group
NK1.1 deletion
supplied antigen
rectal stimulation
A
-
BSA
TNBS...
Embodiment 2
[0259] NK1.1+ lymphocytes have increased CD4+IL4+ / CD4+IFNγ+ ratio in tolerized mice and decreased CD4+IL4+ / CD4+IFNγ+ ratio in non-tolerized mice with experimental colitis
[0260] Tolerized mice
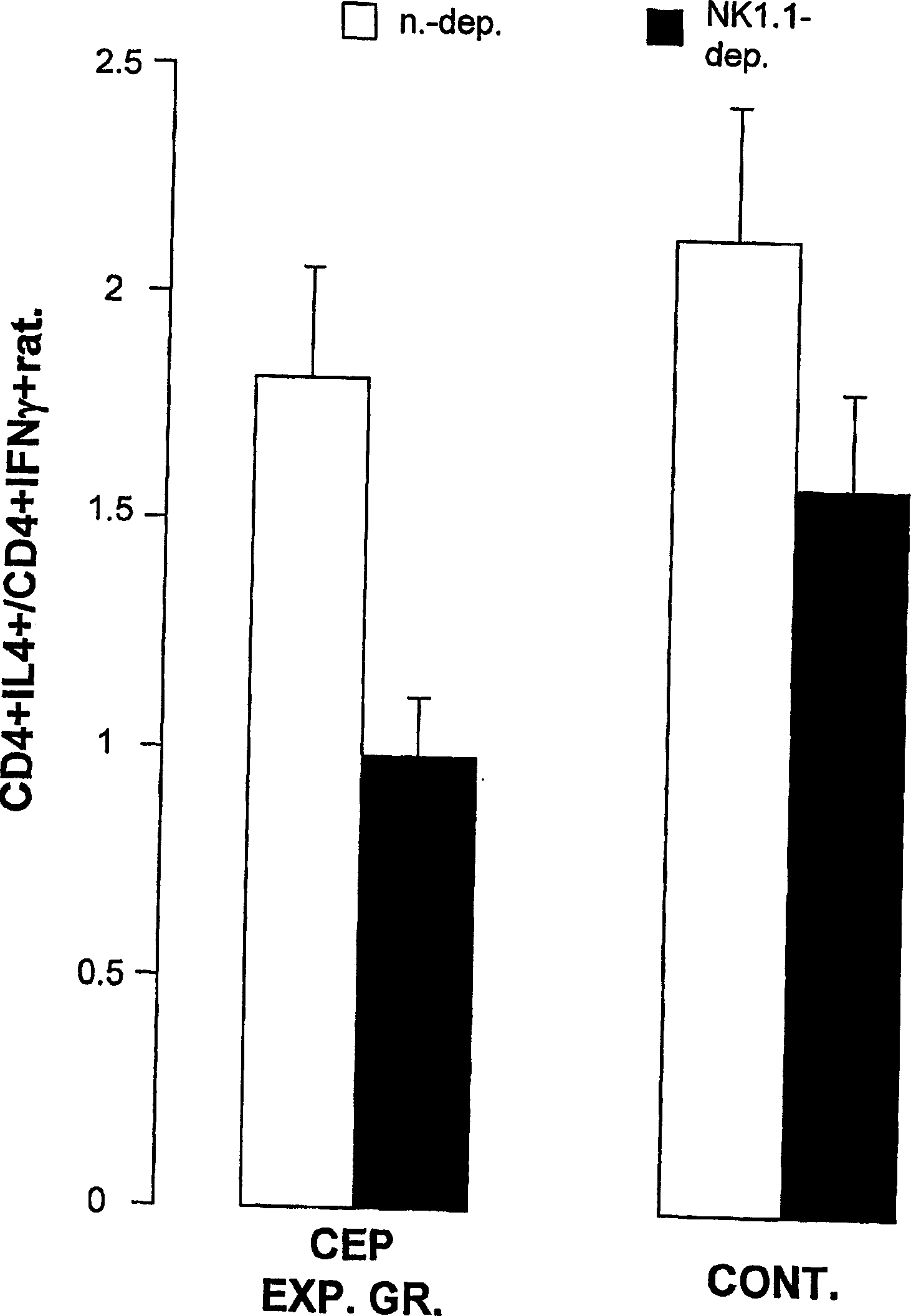
[0261] To study the role of NK1.1+ lymphocytes in tolerized mice, splenocytes and liver-associated lymphocytes (2.5 × 10 6 Splenocytes and 0.5 x 10 6 LAL) and cultured for 72 hours in the presence of CEP and APC. Flow cytometry analysis showed that NK1.1-deficient concomitant oral tolerance-induced decreased CD4+IL4+ / CD4+IFNγ+ ratio compared to non-NK1.1 LAL-depleted tolerized mice (in E and 0.99±0.03 vs. 1.8±0.35 CD4+IL4+ / CD4+IFNγ+ in group B, p figure 2 ). The control NK1.1-deleted group (Group F) revealed a decrease in the CD4+IL4+ / CD4+IFNγ+ ratio compared to the non-NK1.1-deleted group C (2.13±0.36 vs. 1.6±0.29 for C and F groups, respectively ).
[0262] non-tolerized mice
[0263] In contrast to the tolerized group, NK1.1-deletion had the opposite effect on non-tolerized ...
Embodiment 3
[0266] Effects of in vitro sensitization and effect of disease-directed antigens on the ratio of CD4+IL4+ / CD4+IFNγ+ in tolerized and non-tolerized mice with experimental colitis
[0267] To assess the effect of ex vivo exposure to disease-directed antigens on the CD4+IL4+ / CD4+IFNγ+ ratio, splenocytes and liver-associated lymphocytes (2.5 × 10 6 ) splenocytes and (0.5×10 6 ) LAL and cultured for 12 hours in the presence of Con A but in the absence of CEP and APC. Assessments of the effects of NK1.1 deletion in the absence of antigen were similar to those found in the presence of antigen. Lymphocytes harvested from tolerized mice in group B revealed significantly higher CD4+IL4+ / CD4+IFNγ+ ratios compared to NK1.1-null mice in tolerized group E (0.7 ± 0.02 vs. 1.1±0.02, p Figure 5 ). These results suggest that immune training is achieved in vivo and is not affected by cell-antigen incubation in vitro.
[0268] Similarly, flow cytometry analysis showed that the ratio of CD4+IL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com