Synthesis method of 2-chloro-5-chloromethyl pyridine
A technology of chloromethylpyridine and methylpyridine is applied in the field of synthesis of 2-chloro-5-chloromethylpyridine, and can solve the problems of poor sealing performance, high noise, unsatisfactory yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] The present invention will be further described below in conjunction with embodiment:
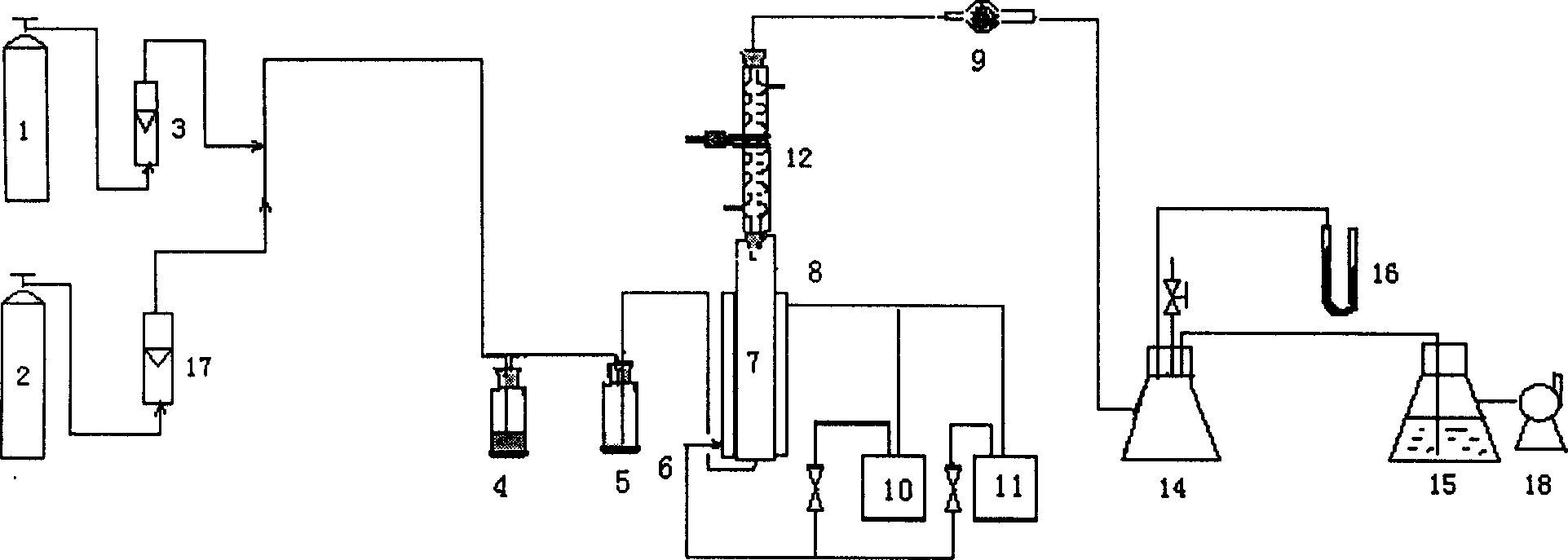
[0014] 1. Add 2-chloro-5-picoline and carbon tetrachloride solvent in the air-lift loop reactor so that the concentration of 2-chloro-5-picoline is 0.33g / ml; the pressure is 745~750mmHg Under certain conditions, dry nitrogen is sprayed into the airlift loop reactor from the bottom of the airlift loop reactor to form an internal circulation flow, and the reactant and solvent are mixed evenly; the catalyst azobisisobutyronitrile is added to make The molar ratio of it to 2-chloro-5-picoline is 1:20, the temperature is raised to 75°C, the solvent is in a reflux state, and nitrogen gas is continued at this temperature for 5 minutes; nitrogen gas flow is stopped, and dry Chlorine gas, make chlorine gas form a liquid internal circulation flow in the air-lift loop reactor, the speed of chlorine gas flow is 300 ~ 400ml / min, and online detection of 2-chloro-5-picoline and 2-chloro-5 - the pur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com