Pterocarya stenoptera extract, its preparation and use
A kind of use and compound technology, applied in the field of maplene and its preparation, can solve the problem of no natural diphenylepoxyheptane compounds, etc., and achieve the effect of inhibiting the cell cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Separation and purification of compound I
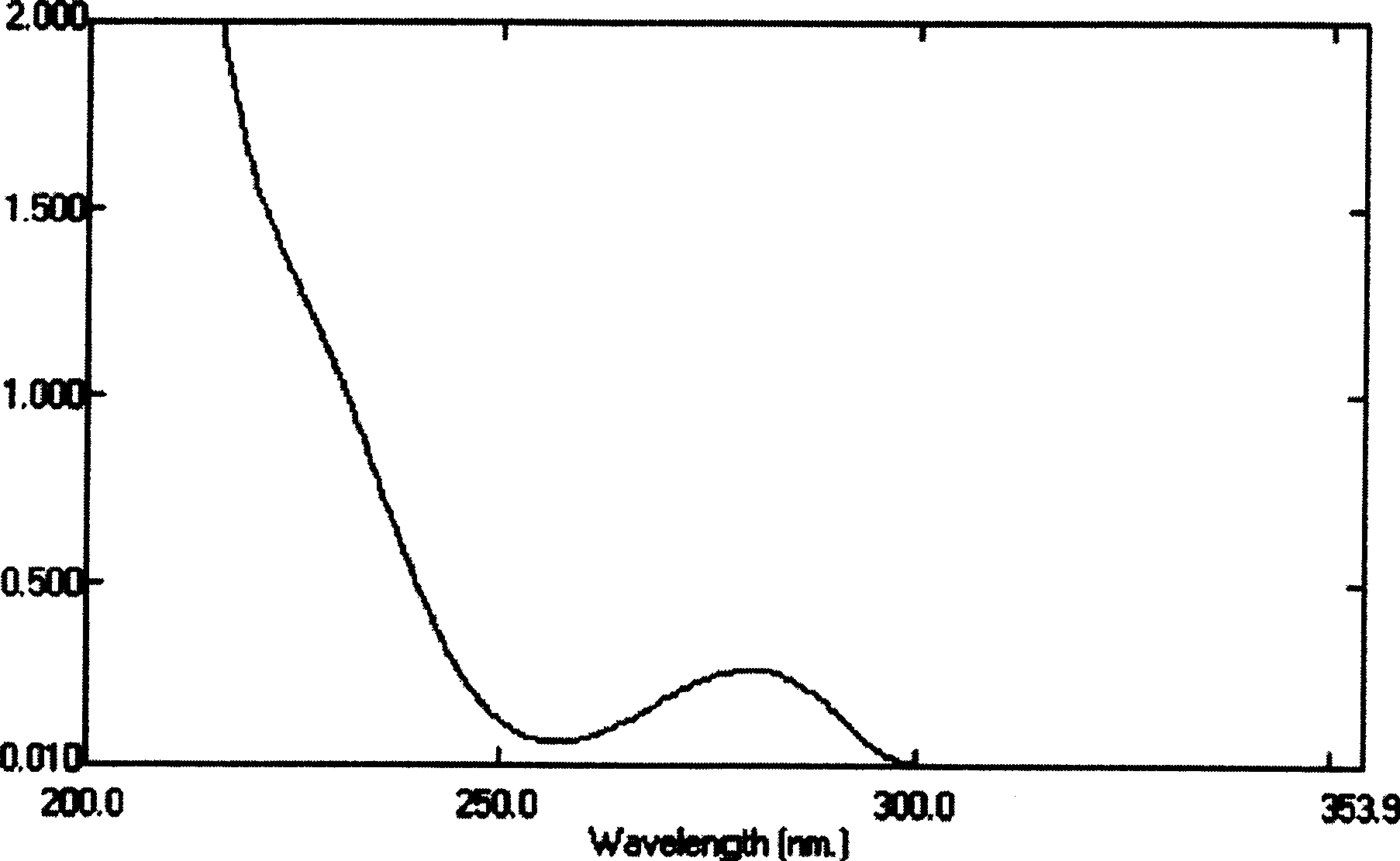
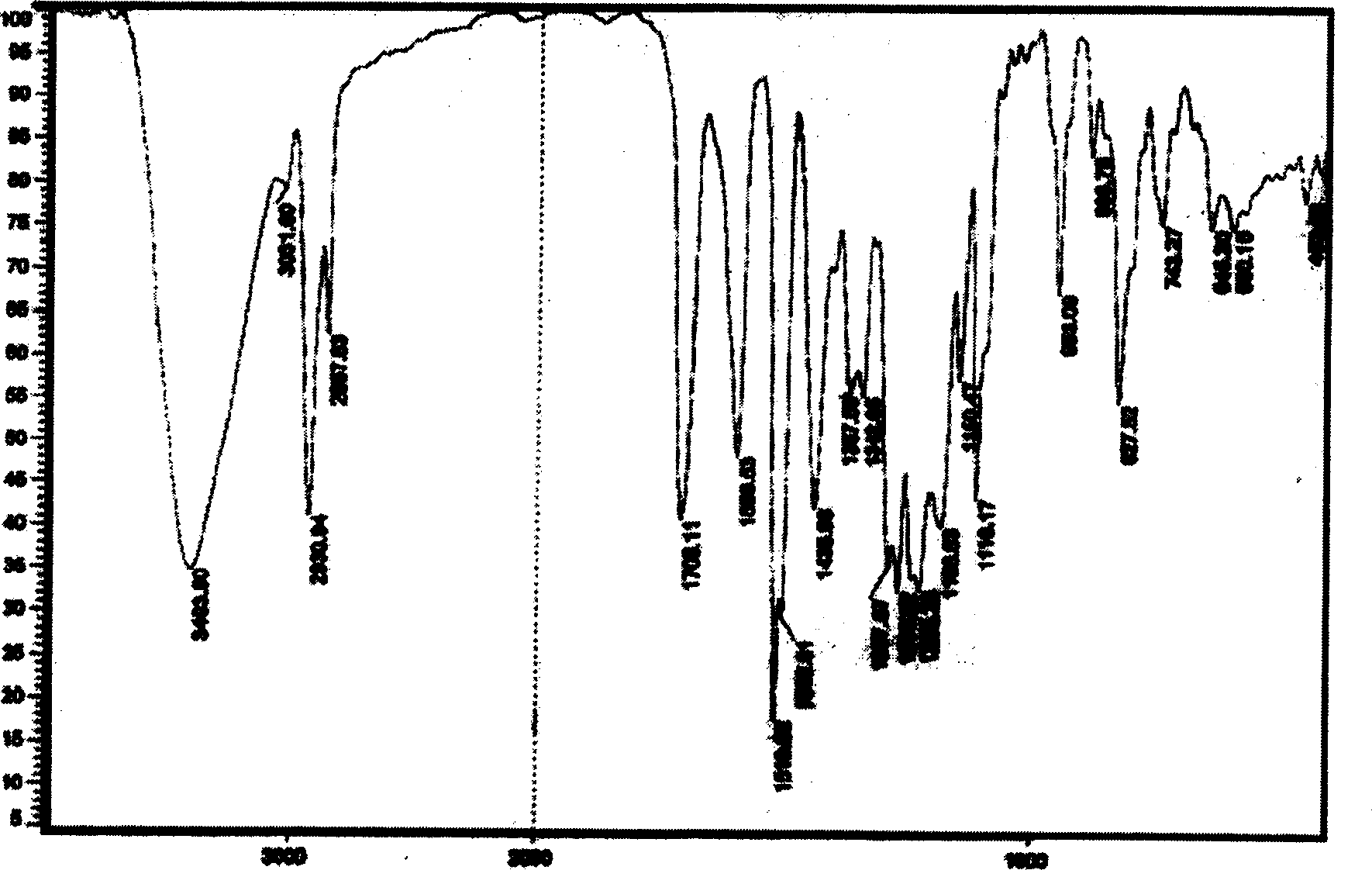
[0021]
[0022] Compound I
[0023] In the formula, the Arabic numerals are the positions of the carbon atoms in the chemical structure.
[0024] Get the dry stem bark (3.5 kg) of Tokyo maple poplar and pulverize it, soak it with 10 liters of 60% ethanol at room temperature for 6 days, filter the aqueous ethanol solution (the same extraction is carried out 8 times), combine the extracts, and concentrate under reduced pressure until it does not contain Ethanol (about 2 liters), extracted 4 times with an equal volume of chloroform, combined the chloroform extracts, concentrated and dried to obtain 10.4 g of chloroform extracts.
[0025] Get the chloroform extract (10.4 grams), add 10 grams of thin-layer chromatography silica gel G after dissolving with appropriate amount of chloroform and mix the sample, use 194 grams of silica gel H dry method to go up the decompression column, use chloroform→metha...
Embodiment 2
[0058] Example 2 Tests for Inhibitory Activity and Apoptosis-Inducing Activity of Cancer Cell Proliferation and Cell Cycle
[0059] 1 Experimental samples and experimental methods
[0060] Preparation of the tested sample solution Take the pure compound I isolated and refined in the above-mentioned Example 1, accurately weigh an appropriate amount, and prepare a solution of the required concentration with methanol for testing the activity.
[0061] Cell lines and cell culture The activity test uses mammalian cancer cell lines such as mouse breast cancer tsFT210 cells, human chronic myelogenous leukemia K562 cells and human colorectal cancer HCT-15 cells. All kinds of cells were subcultured in RPMI-1640 medium containing 10% FBS at 32°C (tsFT210 cells) or 37°C (K562 cells and HCT-15 cells) in an incubator filled with 5% carbon dioxide.
[0062] Cell Proliferation Inhibitory Activity Test Method (SRB Method)
[0063] This method adopts the SRB (sulforhodamine B, Lissamine rhod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com