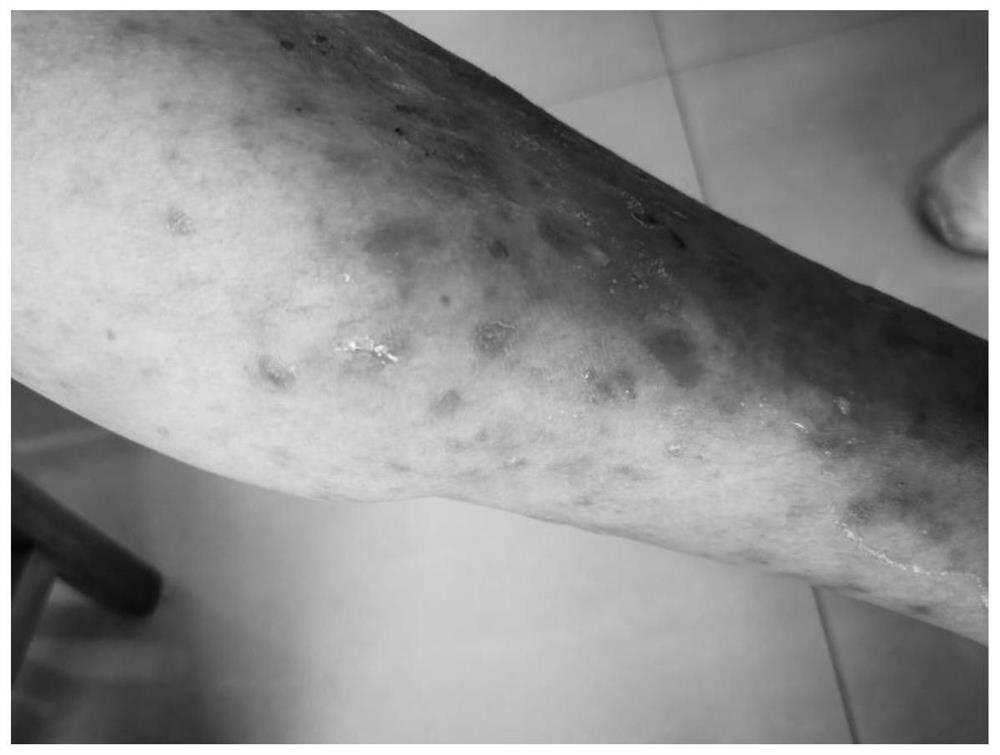
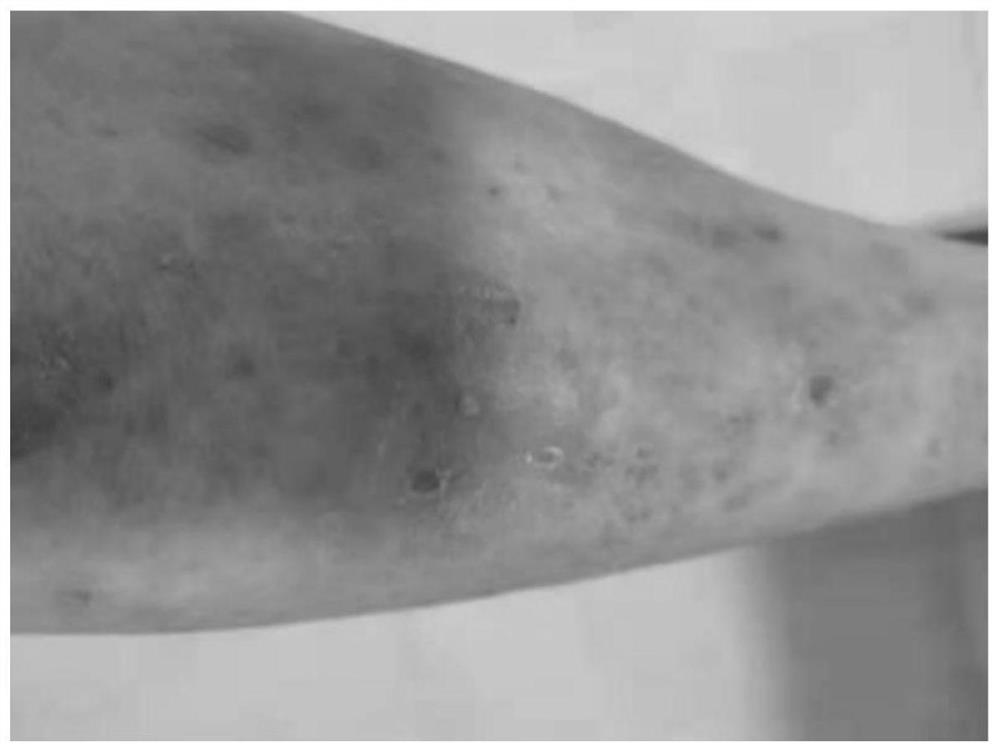
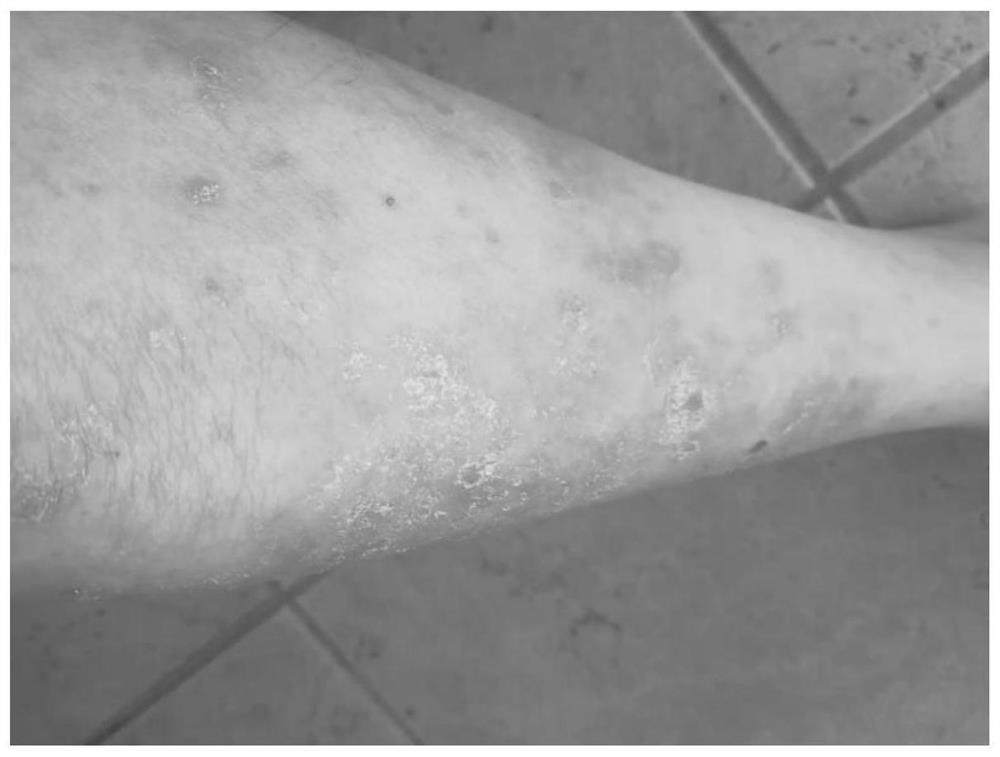
Application and method of superoxide dismutase in preparation of medicine for treating psoriasis
A technology of superoxide and dismutase, applied in biochemical equipment and methods, oxidoreductase, drug combination, etc., can solve the problems of ineffective and difficult to apply psoriasis treatment, etc., to inhibit cell cycle, The effect of reducing the degree of skin keratinization and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The preparation method of medicine for treating psoriasis, concrete steps are as follows:
[0066] (1) Prepare 2× aqueous matrix components: Add glycerin and propylene glycol to deionized water sterilized at 121°C for 15 minutes so that the concentrations of the two are 2.0% and 1.0%, respectively, and denote it as "A";
[0067] (2) Prepare 10× sodium hyaluronate components: dissolve sodium hyaluronate in deionized water sterilized at 121°C for 15 minutes to make the concentration of sodium hyaluronate 2.5%, and use 0.45uM microporous filter membrane, denoted as "B";
[0068] (3) Prepare 100× potassium sorbate component: dissolve potassium sorbate in deionized water sterilized at 121°C for 15 minutes to make the final concentration 10%, and filter with a 0.45uM microporous membrane. Denoted as "C";
[0069] (4) Prepare 200× myristoyl hexapeptide components: dissolve myristoyl hexapeptide in deionized water sterilized at 121°C for 15 minutes to make the final concentra...
Embodiment 2
[0092] The preparation method of medicine for treating psoriasis is as follows:
[0093] (1) Prepare 2× aqueous matrix components: Add glycerin and propylene glycol to deionized water sterilized at 115°C for 20 minutes so that the concentrations of the two are 2.0% and 1.0% respectively, and denoted as "A";
[0094] (2) Prepare 10× sodium hyaluronate components: dissolve sodium hyaluronate in deionized water sterilized at 115°C for 20 minutes to make the concentration of sodium hyaluronate 2.5%, and use 0.45uM microporous filter membrane, denoted as "B";
[0095] (3) Prepare 100× potassium sorbate component: dissolve potassium sorbate in deionized water sterilized at 115°C for 20 minutes to make the final concentration 10%, and filter with a 0.45uM microporous membrane. Denoted as "C";
[0096] (4) Prepare 200× myristoyl hexapeptide components: dissolve myristoyl hexapeptide in deionized water sterilized at 115°C for 20 minutes to make the final concentration 1 mg / ml, which ...
Embodiment 3
[0107] The preparation method of medicine for treating psoriasis is as follows:
[0108] (1) Prepare 2× aqueous matrix components: Add glycerin and propylene glycol to deionized water sterilized at 125°C for 12 minutes so that the concentrations of the two are 2.0% and 1.0%, respectively, and this component is recorded as " Component A";
[0109] (2) Prepare 10× sodium hyaluronate components: dissolve sodium hyaluronate in deionized water sterilized at 125°C for 12 minutes to make the concentration of sodium hyaluronate 2.5%, and use 0.45uM microporous filter membrane, this component is recorded as "component B";
[0110] (3) Prepare 100× potassium sorbate component: dissolve potassium sorbate in deionized water sterilized at 125°C for 12 minutes to make the final concentration 10%, and filter with a 0.45uM microporous membrane. This component is denoted as "Component C";
[0111] (4) Prepare 200× myristoyl hexapeptide component: dissolve myristoyl hexapeptide in deionized ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com