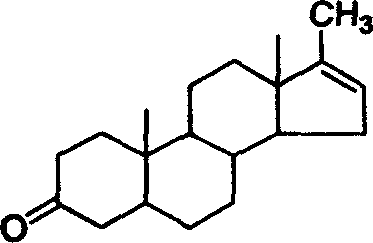
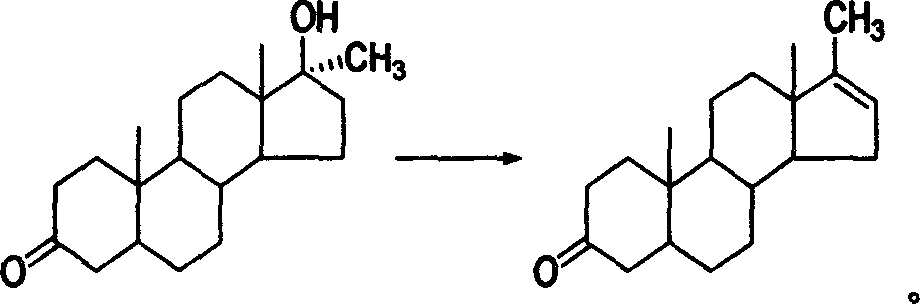
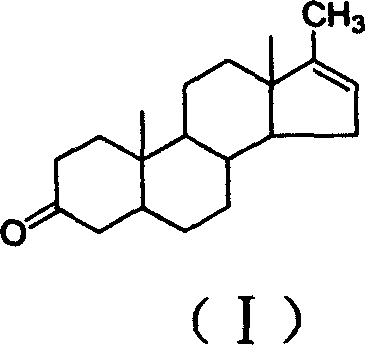
17-methyl-5 alpha-androstane-16-olefin-3-ketone and preparation method
A technology of methylandrosterone and methyl, which is applied in the field of organic chemical synthesis, achieves the effects of high synthesis purity, convenient operation and ingenious utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of Methylandrosterone Using Minandrolone as Raw Material
[0019] Dissolve 100 g of metandrolone in 100 mL of acetic anhydride, add 5 g of phosphoric acid, and stir at 60° C. for 1 hour. After the reaction was completed, it was poured into 5 L of water under stirring, allowed to stand, and filtered to obtain 93 g of a crude product of methylandrosterone. The crude product was recrystallized from ethanol to obtain 85 g of methylandrosterone. MS(m / z)286(M + ); m.p.143~144°C; Assay: 99.7% (HPLC).
Embodiment 2
[0021] Preparation of Methylandrosterone Using Minandrolone as Raw Material
[0022] Dissolve 100 g of metandrolone in 100 mL of propionic anhydride, add 5 g of p-toluenesulfonic acid, and stir at 80° C. for 1 hour. After the reaction was completed, it was poured into 5 L of water under stirring, allowed to stand, and filtered to obtain 93 g of a crude product of methylandrosterone. The crude product was recrystallized from ethanol to obtain 85 g of methylandrosterone. MS(m / z)286(M + ); m.p.143~144°C; Assay: 99.7% (HPLC).
Embodiment 3
[0024] Preparation of Methylandrosterone Using Minandrolone as Raw Material
[0025] Dissolve 100 g of metandrolone in 100 mL of heptanoic anhydride, add 5 g of phosphoric acid, and stir at 60-80°C for 1 hour. After the reaction was completed, it was poured into 5 L of water under stirring, allowed to stand, and filtered to obtain 93 g of a crude product of methylandrosterone. The crude product was recrystallized from ethanol to obtain 85 g of methylandrosterone. MS(m / z)286(M + ); m.p.143~144°C; Assay: 99.7% (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com