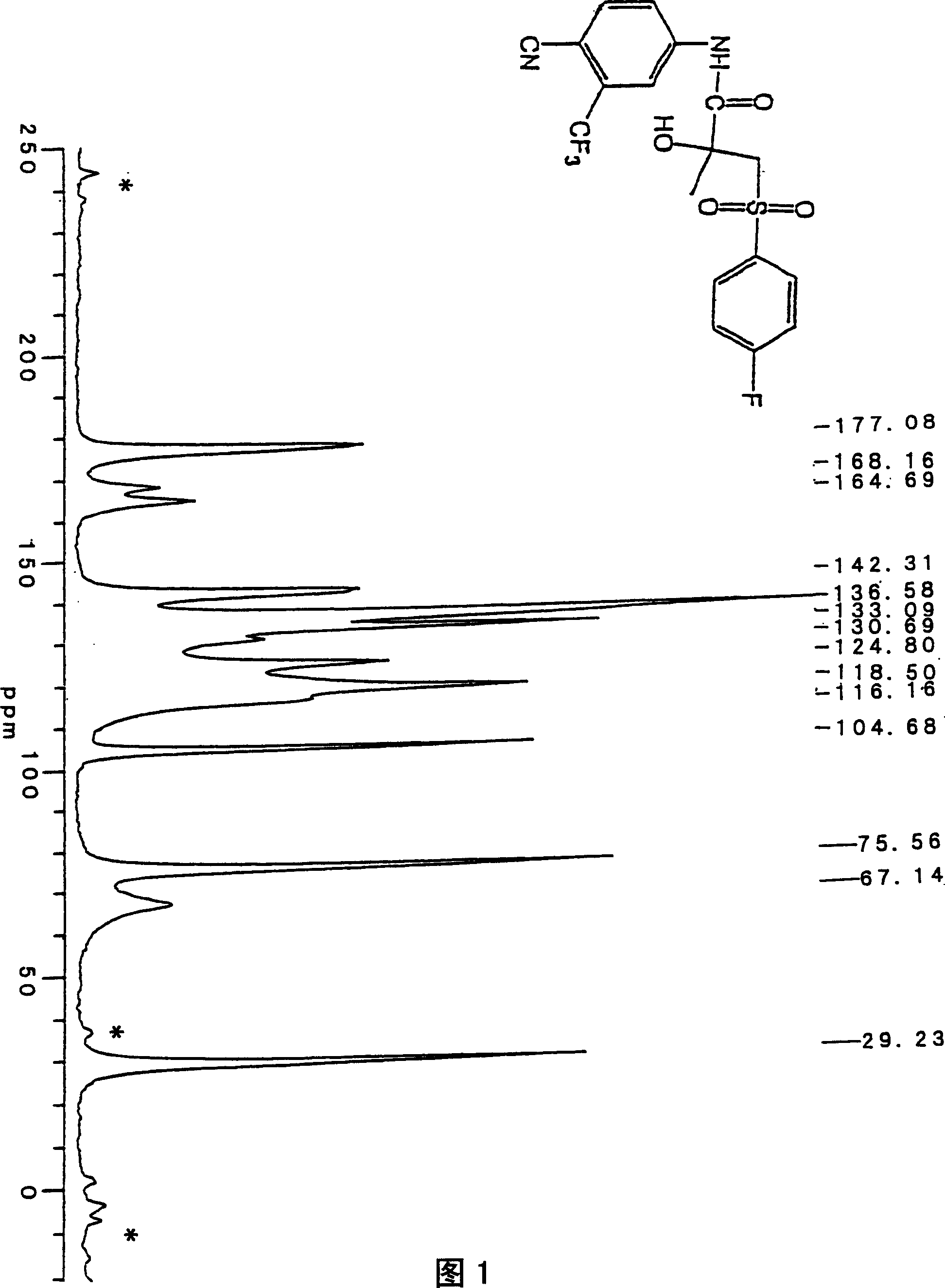
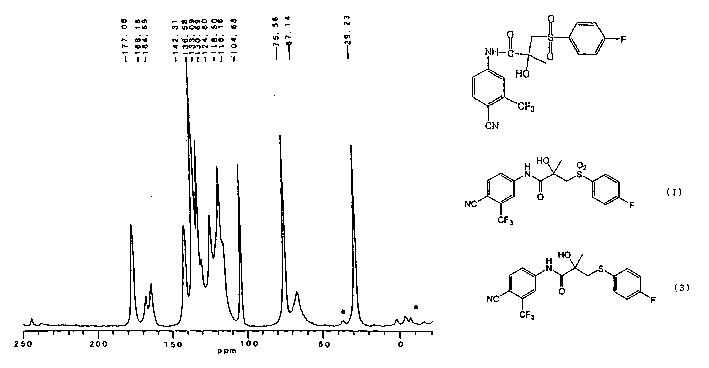
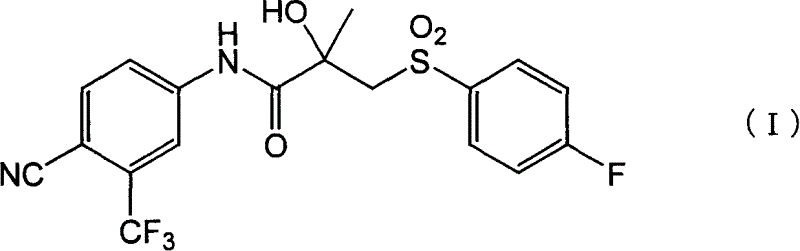
Crystals of bicalutamide and process for their production
A manufacturing method and technology of bicalutamide, applied in the directions of organic chemistry methods, chemical instruments and methods, pharmaceutical formulations, etc., can solve problems such as being unsuitable for industrial production of bicalutamide, not economical, expensive, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0200] Preparation method of bicalutamide crystal
[0201] In the present invention, the method for preparing bicalutamide crystals is characterized in that the method comprises the following steps I-III:
[0202] 1, the preparation step of the solution containing bicalutamide,
[0203] II. the step of adding a hydrocarbon solvent to the solution obtained in step I when required, and
[0204] III. A step of cooling the solution obtained in step I or II to precipitate bicalutamide crystals.
[0205] Step I
[0206] In step I prepare a bicalutamide-containing solution.
[0207] As for the method of preparing the bicalutamide-containing solution, there may be mentioned, for example, a method of adding a solvent to bicalutamide.
[0208] Solvents that can be exemplified include organic solvents such as ethyl acetate, and ethyl acetate is preferred from the viewpoint of solubility.
[0209] The amount of solvent added per 1 g of bicalutamide is generally 1.0ml-10ml, preferably...
reference example 1
[0232] Preparation of monoperoxyphthalic acid
[0233] Deionized water (125ml), Na 2 CO 3 (31.0g, 0.25mol) and 35% H 2 o 2 (29.15 g, 0.3 mol) were successively charged into 500 ml four-necked flasks, and the mixture was stirred in a dry ice-methanol bath at -5°C to 0°C. Phthalic anhydride (37.0 g, 0.25 mol) was added thereto, and the mixture was stirred for 30 minutes. The bath was removed, ethyl acetate (100ml) was added to the mixture, and 98% HO was diluted with deionized water (50ml). 2 SO 4 (15ml) to neutralize the reaction system. After separating the layers, the aqueous layer was extracted with ethyl acetate (60ml). The obtained organic layer (0.64 g) was taken out, and saturated NaI-IPA (isopropanol) solution (5 ml) and 10% acetic acid-IPA solution (20 ml) were added thereto. Boil the mixture for 5 minutes. Titrate with 0.1N aqueous sodium thiosulfate solution. As a result, there was 33.5 g of monoperoxyphthalic acid, and the yield was 76.9%.
reference example 2
[0235] Synthesis of N-methacryloyl-4-cyano-3-trifluoromethylaniline
[0236] The title compound was prepared according to the method described in J. Med. Chem., 1988, 954-959, using 4-cyano-3-trifluoromethylaniline and methacryloyl chloride as starting materials.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com