Novel effectors of dipeptidyl peptidase IV
A dipeptidyl peptidase and effector technology, applied in the field of new effectors of dipeptidyl peptidase IV, can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] In the present invention, oral administration of high-affinity low-molecular-weight enzyme inhibitors is an economical alternative to widely used surgical techniques in the treatment of pathological conditions. Through chemical design for stability, transport and clearance properties, the mode of action can be adjusted and adapted to the characteristics of different individuals.
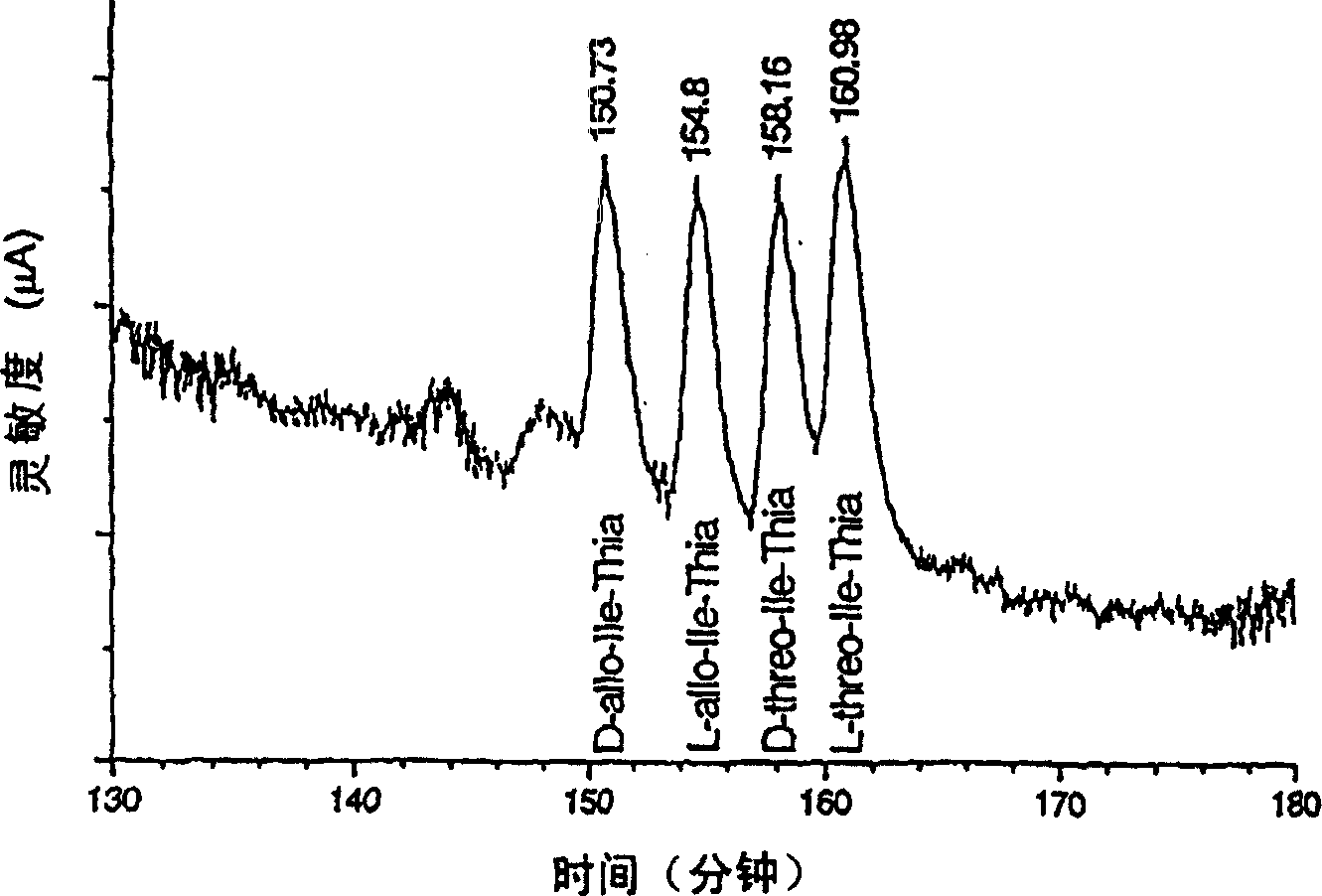
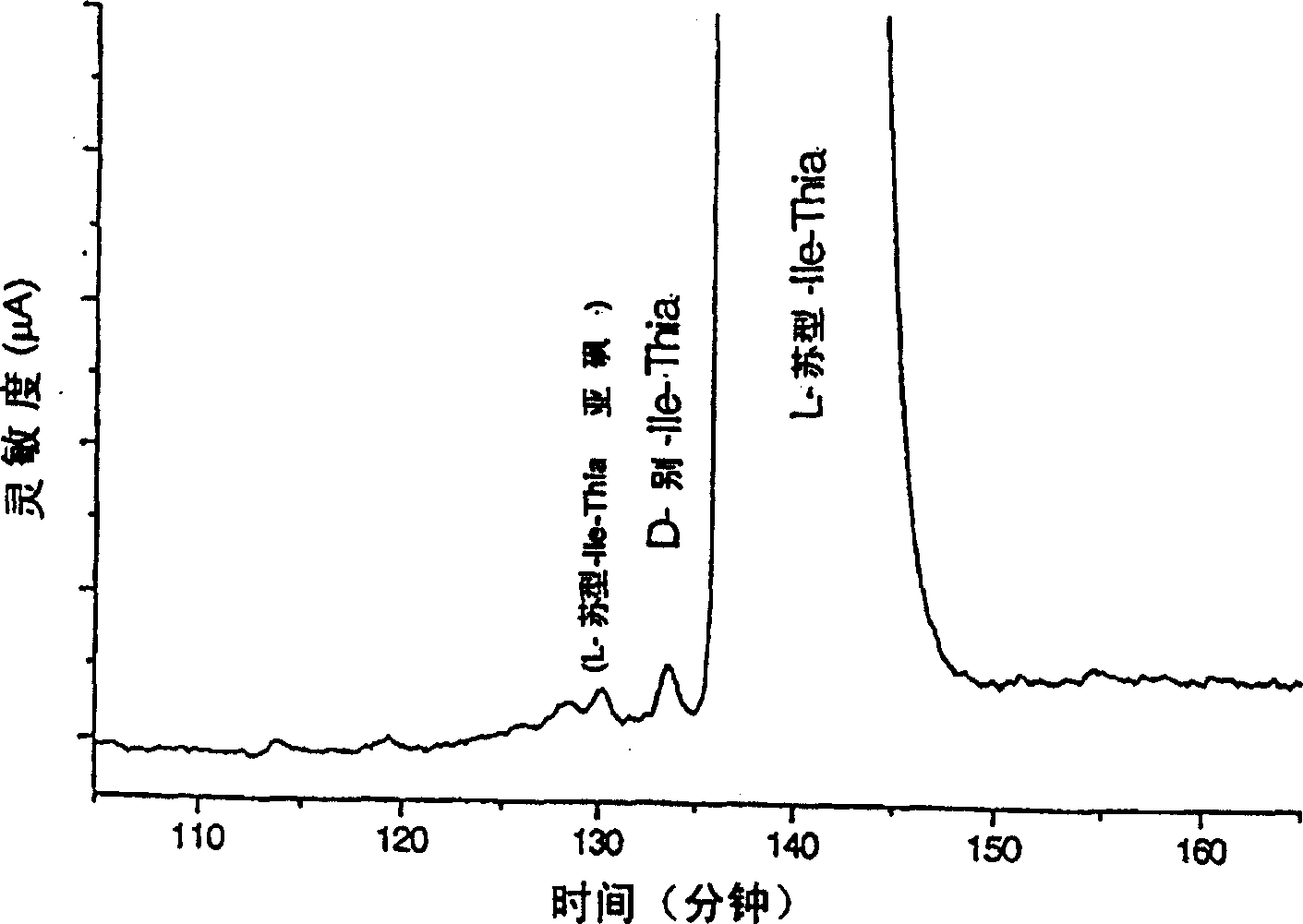
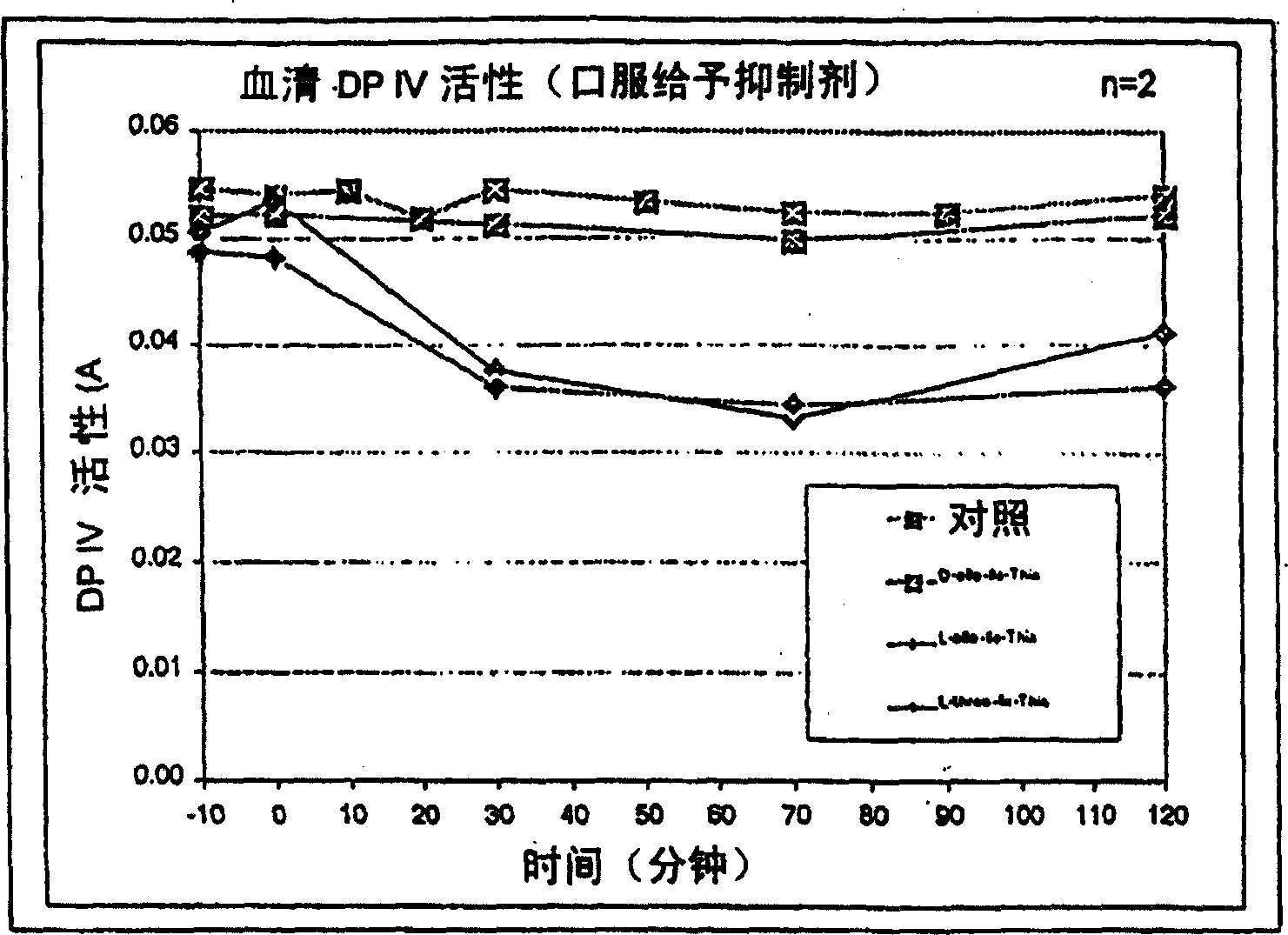
[0031] As mentioned above, for example in the case of long-term treatment of diabetes, it may be necessary to provide effectors with said activity which can meet the different requirements of the patients and treat their symptoms. The said dipeptide compound of the present invention shows that at a (dipeptide compound) concentration of 10 micromolar, especially under the conditions shown in Table 1, dipeptidyl peptidase IV or class DP IV enzyme activity is reduced by at least 10%, especially is at least 40% lower. Usually a reduction of at least 60% or 70% is also required. Preferred effecto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com