Shiff base derivative of carboxymerhyl chitosan and preparation method
A technology of carboxymethyl chitosan Schiff base and carboxymethyl chitosan, which is applied in the field of organic molecular compounds, can solve the problems of enhancing the biological activity of chitosan, and achieves the enhancement of biological activity, water solubility, and good antibacterial properties. Bacteriostatic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 3g polymer carboxymethyl chitosan (Mw=7.0×10 5 ) into 100ml of 95% ethanol, mixed with 10ml of 5-nitrosalicylaldehyde, refluxed at 80°C for 6 hours, filtered, washed with ethanol, extracted with absolute ethanol in a Soxhlet extractor for 24 hours, and vacuum freeze-dried , that is to obtain high molecular weight 2-(2-hydroxy-5-nitro-phenylimino)-6-carboxymethyl chitosan.
Embodiment 2
[0030] 3g low molecular carboxymethyl chitosan (Mw=1.0×10 4 ) into 100ml of 95% ethanol, mixed with 10ml of 5-nitrosalicylaldehyde, refluxed at 80°C for 10 hours, filtered, washed with ethanol, extracted with absolute ethanol in a Soxhlet extractor for 24 hours, and vacuum freeze-dried , that is, low molecular weight 2-(2-hydroxyl-5-nitro-phenylimino)-6-carboxymethyl chitosan was obtained.
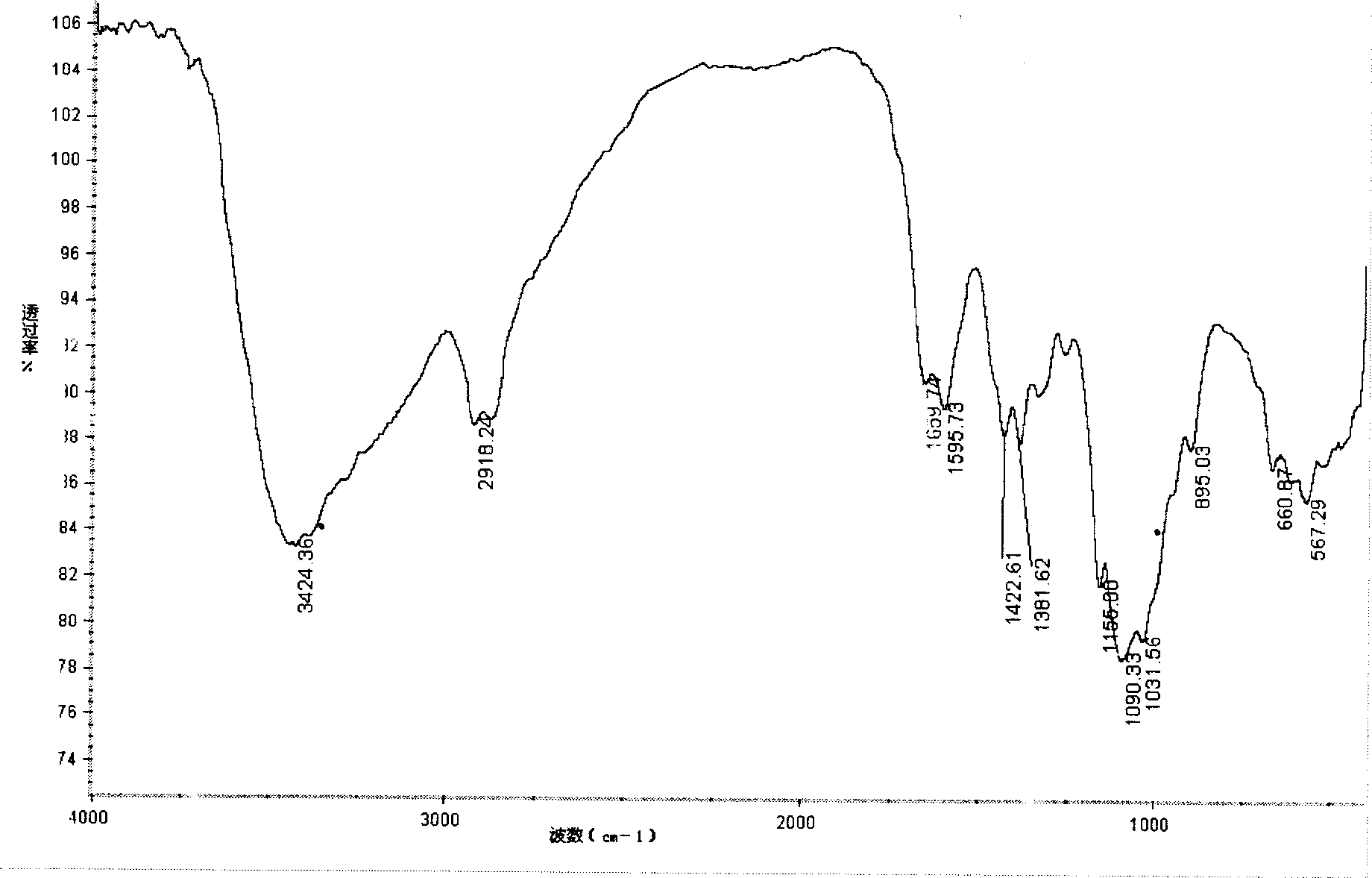
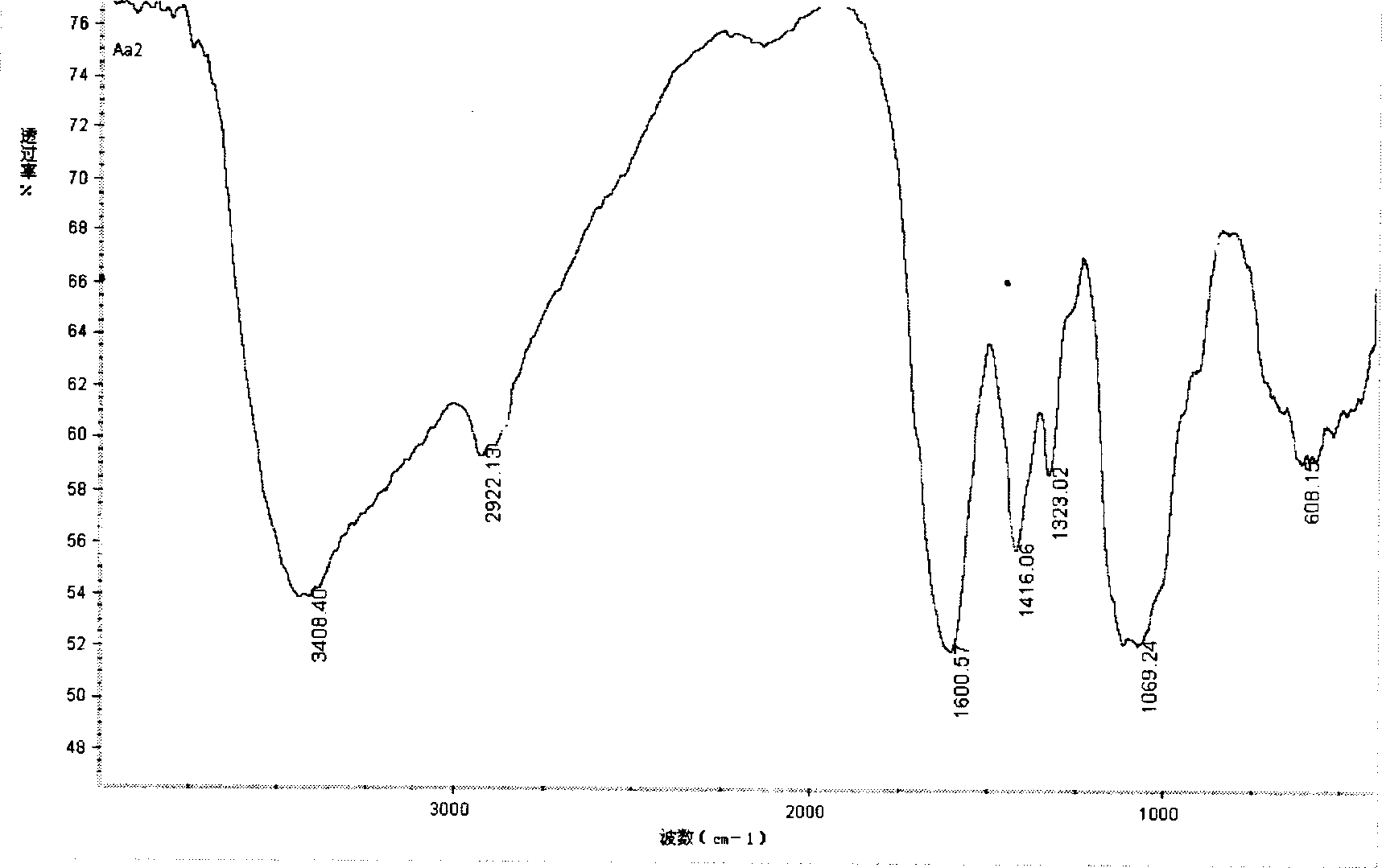
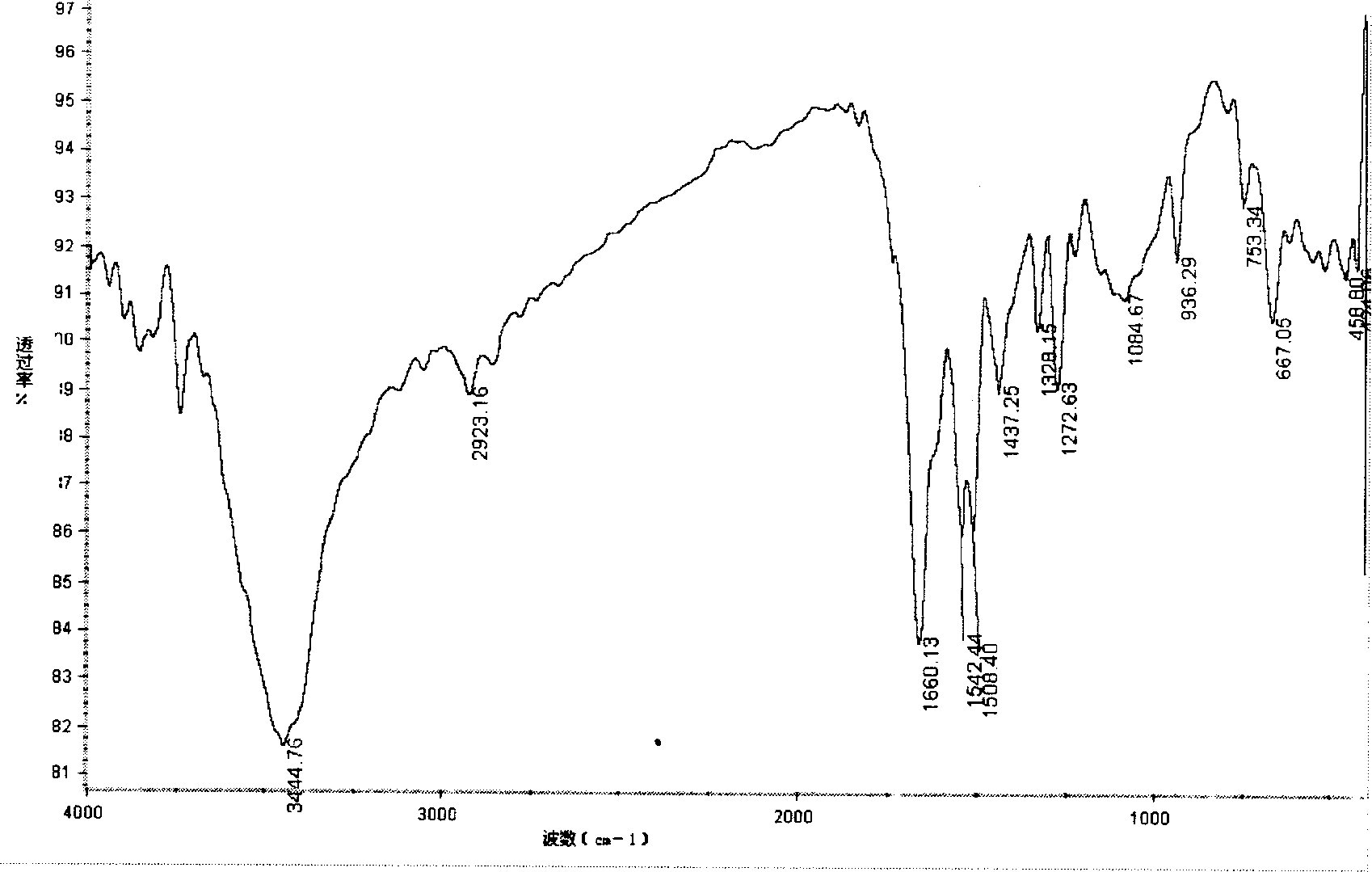
[0031] The compound was identified by infrared spectroscopy, as image 3 As shown, 3444.76cm -1 Left and right are stretching vibration peaks of O-H and N-H, 2923.16cm -1 Left and right are the stretching vibration peaks of C-H, at 1660.13cm -1 The absorption at is obviously stronger than the carbonyl group of chitosan, which is the absorption peak of imine group, 1508.40cm -1 and 1542.44cm -1 The absorption peaks of the benzene ring and the nitro group are at the peak, which proves the introduction of the phenyl group and the formation of the Schiff base. The derivative is easily so...
Embodiment 3
[0033] 3g polymer carboxymethyl chitosan (Mw=7.0×10 5 ) was added in 100ml of 80% ethanol, mixed with 17ml of 5-chloro salicylaldehyde, refluxed at 80°C for 6 hours and then filtered. After the product was washed with ethanol, it was extracted with absolute ethanol in a Soxhlet extractor for 72 hours, and vacuum freeze-dried. That is to obtain high molecular weight 2-(2-hydroxy-5-chloro-phenylimino)-6-carboxymethyl chitosan.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com