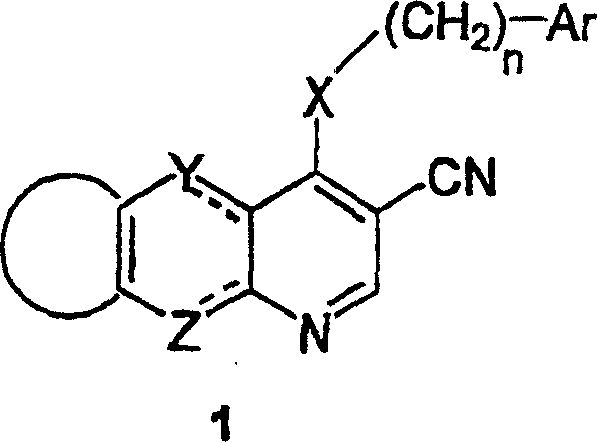
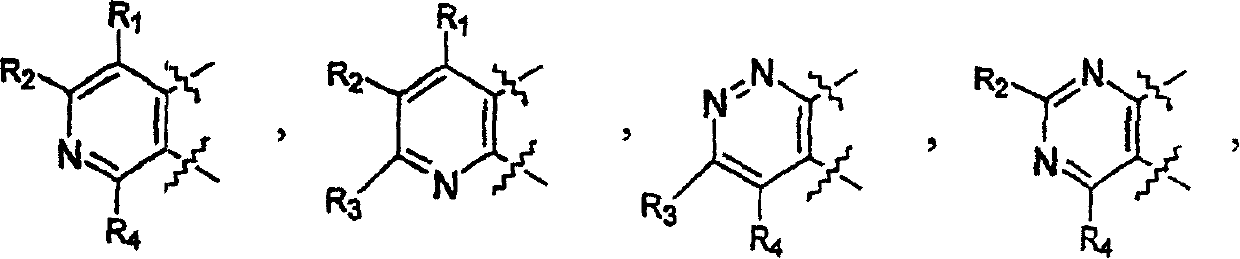
Tricyclic protein kinase inhibitors
A benzene ring and aromatic ring technology, applied in the field of aromatic tricyclic compounds and their pharmaceutically acceptable salts, can solve the problem of no combination of substituents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0786] 4-oxo-1,4-dihydrobenzo[g]quinoline-3-carbonitrile
[0787]A suspension of 5.6 g (30 mmol) of 3-amino-2-naphthoic acid in 30 ml of N,N-dimethylformamide dimethyl acetal was refluxed for 6 hours. Removal of the solvent gave 7.06 g (86.4%) of methyl 3-{[(dimethylamino)methylene]amino}-2-naphthoate as a dark oily residue. To a solution of 20.8 ml (52 mmol) n-butyllithium (2.5M in hexane) in 18 ml tetrahydrofuran (THF) was added dropwise a solution of 5.97 ml (114 mmol) acetonitrile in 100 ml THF at -78°C. After the addition was complete, the suspension was stirred for 15 minutes. To this was added dropwise 7.02 g (26 mmol) of 3-{[(dimethylamino)methylene]amino}-2-naphthoate in 50 ml of THF. The resulting reaction mixture was stirred at -78°C for 1 hour. Then 7.8 g (130 mmol) of acetic acid were added dropwise. The reaction mixture was allowed to warm to room temperature and diluted with water. The precipitate was collected by filtration, washed with water and ethyl ace...
Embodiment 2
[0792] 4-Chlorobenzo[g]quinoline-3-carbonitrile
[0793] 3.5 g (16 mmol) of 4-oxo-1,4-dihydrobenzo[g]quinoline-3-carbonitrile in 35 ml of phosphorus oxychloride and 22 drops of N,N-dimethylformamide (DMF) The reaction mixture was heated at 100-110°C for 5 hours. After cooling, the mixture was concentrated to dryness in vacuo to give a dark residue. The residue was partitioned between dichloromethane and ice-cold saturated aqueous sodium carbonate. The organic layer was washed with ice-cold brine and dried over sodium sulfate. The organic solution was passed through a short column of silica gel, eluting further with additional dichloromethane. Removal of solvent gave 1.89 g (49.5%) of product as a bright yellow solid, mp 253-255°C.
[0794] 1 H NMR (DMSO-d 6 ): δ7.77(m, 2H); 8.33(d, J=9.3, 1H); 8.39(d, J=9.5, 1H); 8.91(s, 1H); 9.08(s, 1H); 9.18(s , 1H). MS (ES, cationic mode): C 14 h 7 ClN 2 Calculated value of m / z: 238.68, measured value: 239.2 (M+H) + .
[0795] ...
Embodiment 3
[0798] 4-(4-Phenoxyanilino)benzo[g]quinoline-3-carbonitrile
[0799] 141.8mg (0.59mmol) 4-chlorobenzo [g] quinoline-3-carbonitrile, 111.1mg (0.60mmol) 4-phenoxyaniline and 57.8mg (0.50mmol) pyridine hydrochloride in 8ml 2- The reaction mixture in ethoxyethanol was heated at 110-120°C for 1 hour. After cooling, the mixture was diluted with water and made basic by adding 125.0 mg (1.18 mmol) of sodium carbonate. The precipitate was collected by filtration and washed with water. Drying in vacuo gave the crude product. The crude product was purified by chromatography eluting with a gradient of dichloromethane / methanol from 100:0 to 86:14 to afford 167.8 mg (73.4%) of pure product as a yellow solid, mp 250-251°C.
[0800] 1 H NMR (DMSO-d 6 ): δ7.05(s, 1H); 7.08(s, 1H); 7.14(m, 3H), 7.43(m, 4H); 7.66(m, 2H), 8.11(d, J=8.1, 1H); 8.19 (d, J = 7.8, 1H); 8.55 (d, 2H); 9.24 (s, 1H); 10.22 (bs, 1H). MS (ES, cationic mode): C 26 h 17 N 3 Calculated m / z of O: 387.44, found: 388.2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com