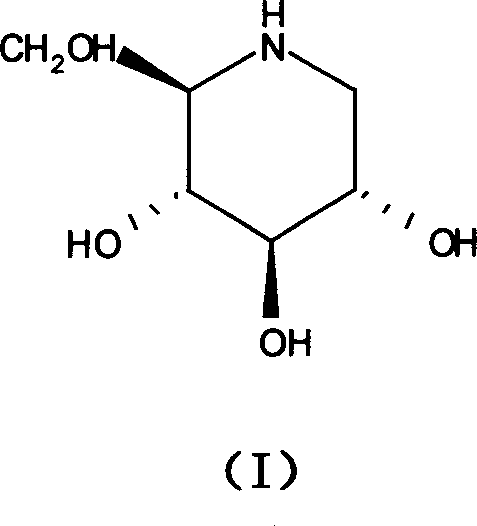
Use of 1-deoxynojirimycin for preparing diabete and disney disease drug
A technology of deoxynojirimycin, diabetic nephropathy, applied in the field of pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
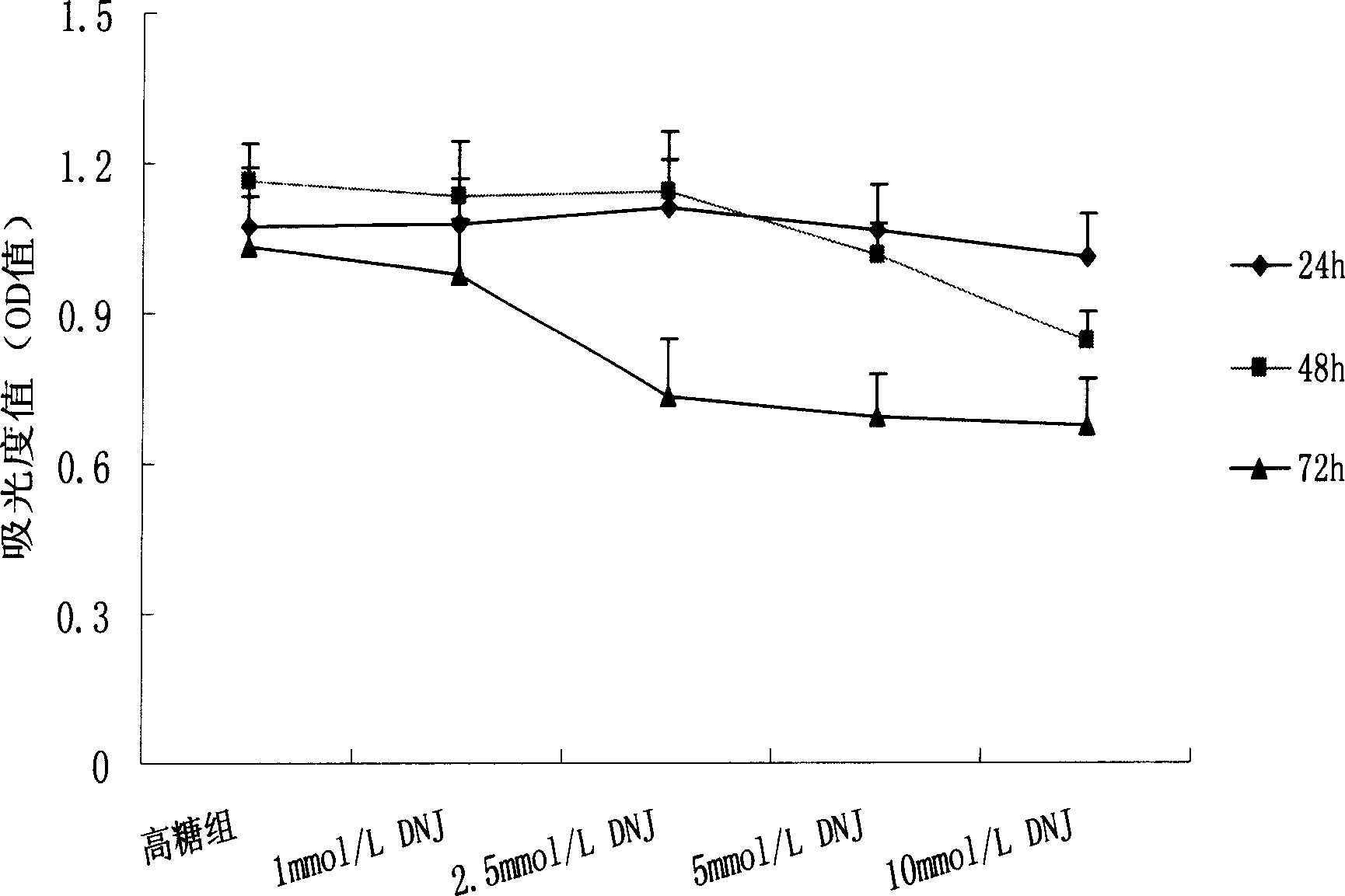
[0027] Example 1 Effect of 1-deoxynojirimycin on proliferation of rat mesangial cells cultured in high glucose
[0028] 1. Materials and Reagents
[0029] The rat mesangial cell line was donated by Professor Mei Changlin, Department of Nephrology, Shanghai Changzheng Hospital Affiliated to Second Military Medical University. Reagents: 1-deoxynojirimycin is a product of Wako, Japan; MTT and DMSO are products of AMRESCO; Trypan Blue is a product of Sigma, USA. The microplate reader was produced by Labsystem MK3 in Finland.
[0030] 2. Method
[0031] 2.1 The effect of high glucose on the proliferation of mesangial cells
[0032] Experimental grouping: normal glucose group (NG): the concentration of D-glucose is 5 mmol / L. High glucose group (HG): D-glucose concentration is 30mmol / L. Each group has 6 replicate wells. Hypertonic group (mannitol group): D-glucose (5mmol / L)+mannitol (25mmol / L).
[0033] Experimental method: Cells were inoculated in 96-well cell culture plates ...
Embodiment 21
[0060] Example 21 - Effect of deoxynojirimycin on the expression of α-SMA in rat mesangial cells cultured in high glucose
[0061] 1. Materials and reagents:
[0062] α-smooth muscle actin Wuhan Boster Biological Company, diluted 1:200 with sterile water when used. Rat type IV collagen (type IV collagen), rat fibronectin (Fibronectin, FN) ELISA kits were purchased from Shanghai Senxiong Biological Co., Ltd. UltraSensitive TM S-P immunohistochemical staining kit, diaminobenzidine (DAB) chromogen, Fuzhou Maixin Biotechnology Co., Ltd.
[0063] 2. Method
[0064] 2.1 Experimental grouping and drug intervention training
[0065] Experimental grouping: ① normal glucose group (NG): 5mmol / L D-glucose; ② high glucose group (HG): 30mmol / L D-glucose; ③ mannitol group (M): 5mmol / L D-glucose + 25mmol / L L mannitol; ④ DNJ group: D-glucose 30mmol / L + DNJ 5mmol / L, and a normal glucose negative control group (without primary antibody).
[0066] Drug intervention time: Immunocytochemical...
Embodiment 3
[0075] Example 3 1-deoxynojirimycin on the synthesis of type IV collagen and fibers in rat mesangial cells cultured in high glucose
[0076] The effect of connexin
[0077] 1. Materials and Reagents
[0078] Rat type IV collagen typeIVcollagen, rat fibronectin (Fibronectin) ELISA kits were purchased from Shanghai Senxiong Technology Industrial Co., Ltd.
[0079] 2. Experimental method
[0080] 2.1 ELISA method to determine the expression of type IV collagen and fibronectin
[0081] MSc cells were inoculated in 24-well plates according to conventional methods, and after being synchronized in the G0 phase, they were divided into normal glucose concentration (NG) group: containing D-glucose 5mmol / L; high glucose concentration (HG) group: containing D-glucose 30mmol / L L; Mannitol group (M) group: D-glucose 5mmol / L+25mmol / L mannitol; DNJ group: D-glucose 30mmol / L+1-deoxynojirimycin (DNJ). After 24h, 48h, and 72h of intervening cells, the supernatant was col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com