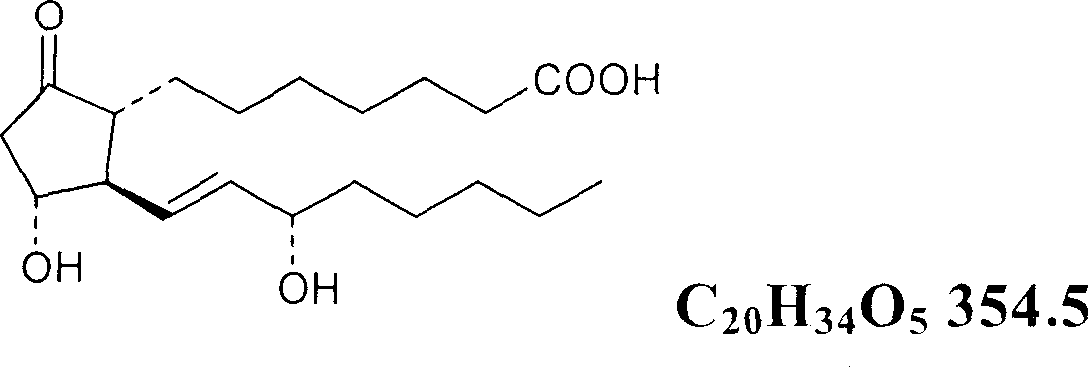
Oral preparation of prostaglandin E1
A preparation and oral technology, applied in the field of sublingual tablets of prostaglandin E1, can solve problems such as inability to take it orally
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1α-CD and PGE 1 Preparation of clathrates:
[0030] Weigh PGE 1 50mg, dissolved in 1ml of absolute ethanol, α-CD 686mg dissolved in 10ml of distilled water, then mixed the two liquids, ultrasonicated for 10 minutes, then placed in a freeze dryer for 24 hours, and the dried product was placed in a vacuum dryer Available in 48 hours. The moisture content of the finally obtained freeze-dried product should be controlled below 1%.
Embodiment 2
[0031] Example 2 HP-β-CD (degree of substitution = 5.0) and PGE 1 Preparation of clathrates:
[0032] Weigh PGE 1 Dissolve 50mg in 1ml of absolute ethanol, dissolve 1.9g of HP-β-CD in 10ml of distilled water, then mix the two liquids, after ultrasonic treatment for 5 minutes, freeze-dry in a freeze dryer for 24 hours, and dry the obtained product in a vacuum Place it in the container for 48 hours. The moisture content of the resulting lyophilizate should be less than 1%.
Embodiment 3
[0033] Example 3 β-CD and PGE 1 Preparation of clathrates:
[0034] Weigh PGE 1 50mg, dissolved in 1ml of absolute ethanol, β-CD 1.6g dissolved in 100ml of distilled water, then mixed the two liquids, after ultrasonic treatment for 5 minutes, placed in a freeze dryer for 24 hours, and the dried product was placed in a vacuum dryer Leave it for 48 hours. The moisture content of the resulting lyophilizate should be less than 1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com