Library of compounds labelled with radioisotope
A radioactive isotope and compound technology, applied in the direction of organic compound library, chemical library, organic compound preparation, etc., can solve the problems of prolonging time and increasing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Example 1 - Incorporation of traces into the ring 14 C Synthesis of Labeled Benzoic Acid Liquid / Solid Phase Libraries
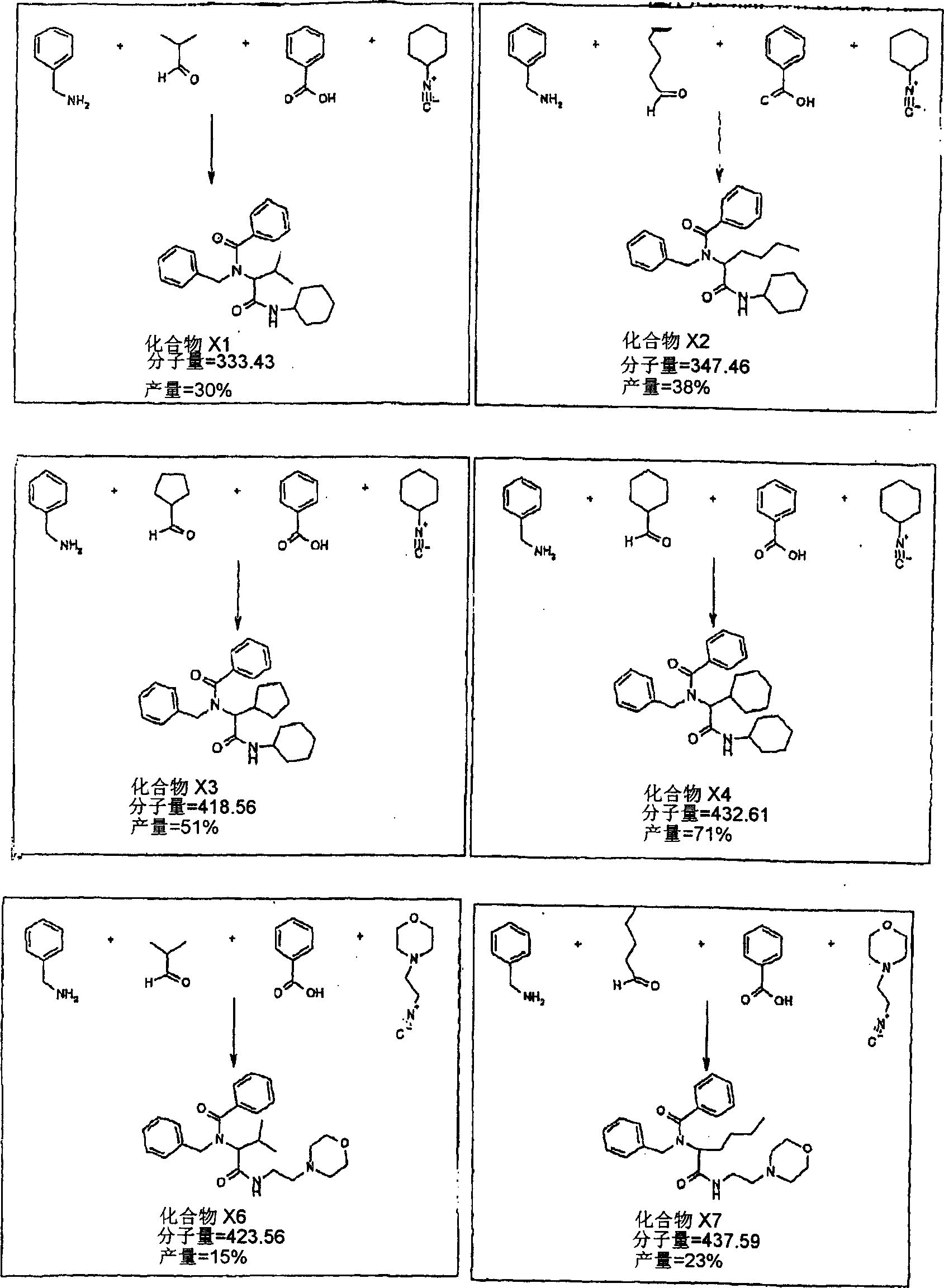
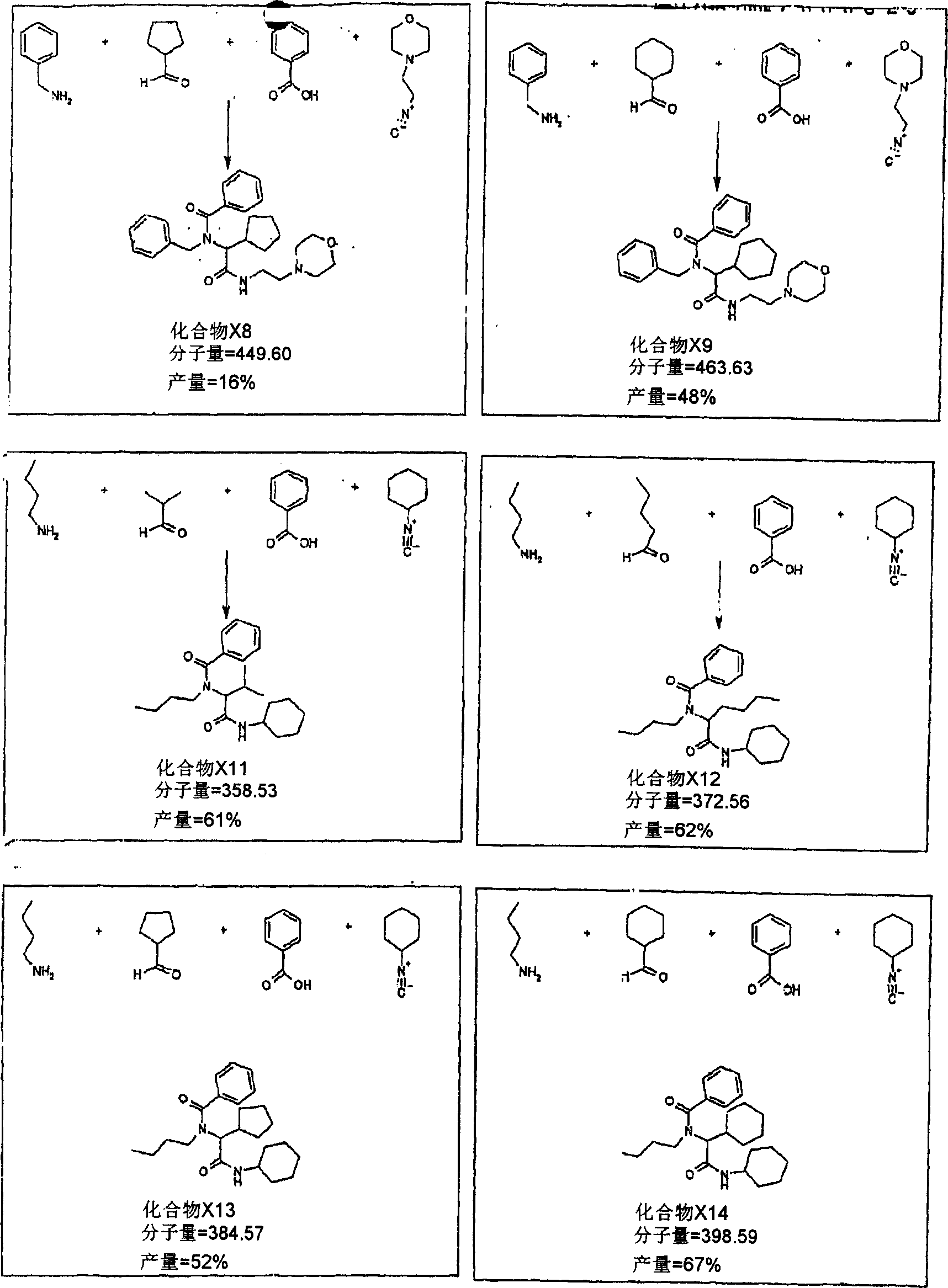
[0130] Typical examples of chemical library products are described in the Ugi reaction (Cao, X., Moran, EJ., Siev, D., Lio, A., Ohashi, C and Mjalli, AMM; 1995., Boorg and Med Chem Lett., 5: 2953-2958; and Nakamura, M., Inoue, J and Yamada, T., 2000., Bioorg and Med Chem Lett., 10: 2807-2810) This reaction can be carried out in liquid or on immobilized resin, including Condensation of 4 reactants, in this case carboxylic acids, amines, aldehydes and isocyanides. The final product (ie, library compound) will vary depending on the different reactants chosen.
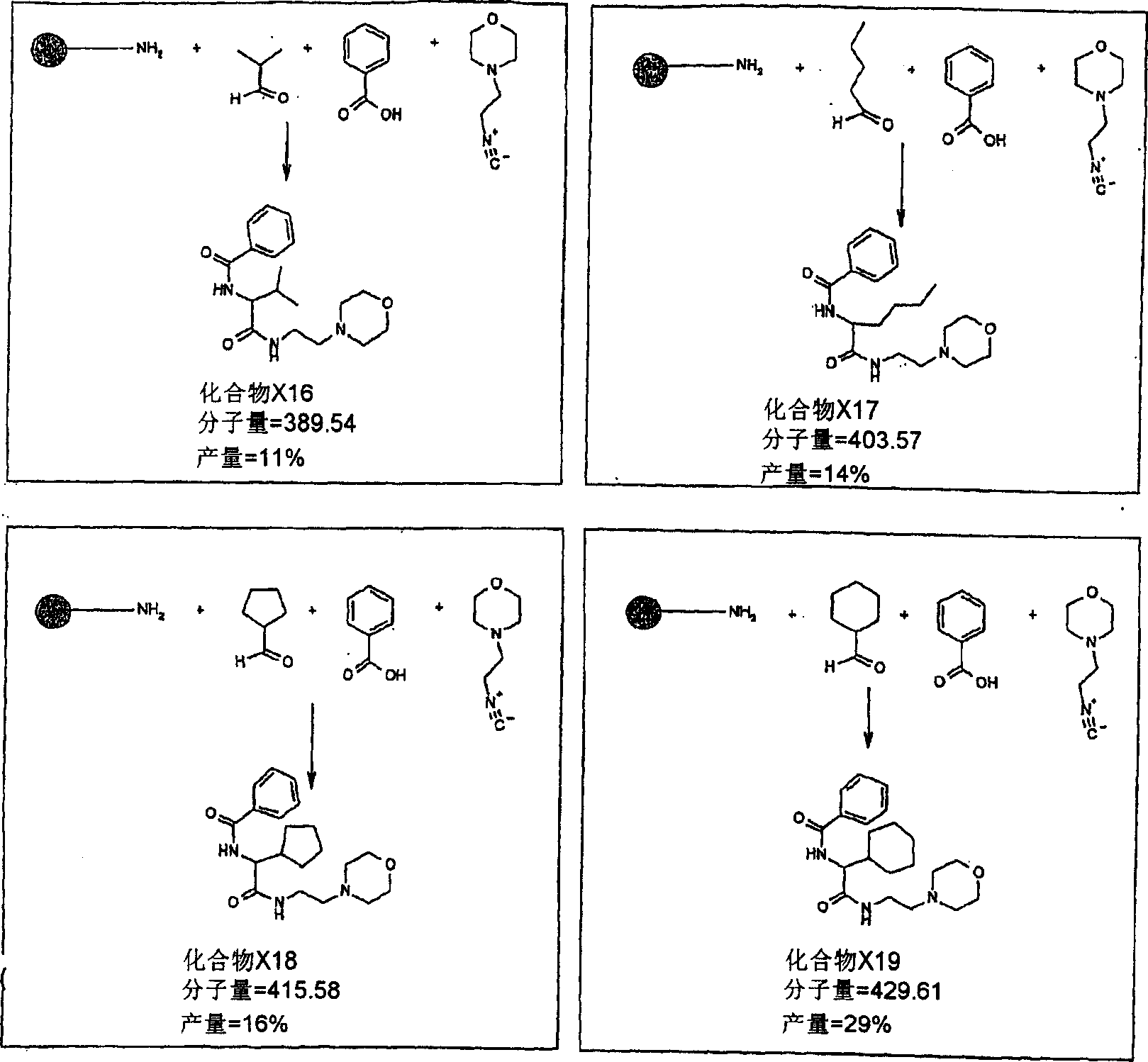
[0131] The following are some exploratory experiments using the mixture of reactants as described in Table 1. Reactions x1-x9 are carried out in liquid. Reactions x21-x29 were performed with TentaGel S-RAM resin. This solid support can donate amino groups for use in the reaction.
[0132]...
Embodiment 1Bx4---x4
[0142] The synthesis of embodiment 1Bx4---x4
[0143] Using the general procedure for 1B above, benzylamine (44ml, 0.4mmol) and cyclopentanecarbaldehyde (48[mu]l, 0.4mmol) were added to anhydrous methanol (0.5ml). The resulting solution was stirred at 28°C for 10 minutes. To this solution was added anhydrous methanol (1ml) 14 C Slightly labeled benzoic acid (from 1A) (48 mg, 0.4 mmol) followed by cyclohexylisocyanide (0.4 mmol). The reaction was stirred at 28°C for 60 hours, and the resulting crude precipitate was filtered, washed with ice-cold methyl alcohol, and dried in vacuo to obtain crude N-benzyl-N-(cyclohexylcyclohexylcarbamoylmethyl)benzamide (122mg, 71%)
[0144] 1 H NMR□ □ (400MHz, CDCl 3 )7.55-6.85(m, 10H), 4.67(d, J 16.2Hz, 1H), 4.45(d, J 16.2Hz, 1H), 4.17(d, J 9.5Hz, 1H), 3.66(m, 1H), 2.41 (m, 1H), 1.95-1.45(m, 10H), 1.40-0.85(m, 10H).
[0145] 13 C NMR □c (400MHz, CDCl 3 )24.6, 25.5, 25.7, 25.7, 26.3, 29.7, 30.2, 32.6, 32.9, 36.1, 47.7, 52.9, 66.7, 126...
Embodiment 1Cx22---x22
[0149] The synthesis of embodiment 1Cx22---x22
[0150] Using the general procedure for 1C above, Rink resin ((1.1 mmol, 0.055 g) was deprotected with 20% piperidine in dichloromethane (DCM) (3 x 1 ml). The resin was dissolved in 50% DCM:MeOH (1 ml) Swell in the medium for 30 minutes. Add valeraldehyde (64 μl, 10 equivalents, according to the initial resin loading) to the pre-swelled resin, and stir the reaction mixture for 10 minutes at 28° C. Add 14 C Mildly labeled benzoic acid (from 1A) (73 mg, 10 equiv) and cyclohexylisocyanide (76 μl, 10 equiv), the resin was stirred at 28°C for 36 hours. The resin was washed with DCM (10 x 5ml) and dried in vacuo. The resin was cleaved with 30% TFA:DCM (1 ml) for 3 hours. The resin was removed by filtration, and the filtrate was concentrated under reduced pressure to obtain crude N-(1-cyclohexylcarbamoylpentyl)benzamide (17.9 mg, 94%).
[0151] 1 H NMR□ □ (400MHz, CDCI 3 ) 7.82 (d, J 7Hz, 2H), 7.54-7.42 (m, 3H), 6.61 (d, J8Hz, 1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com