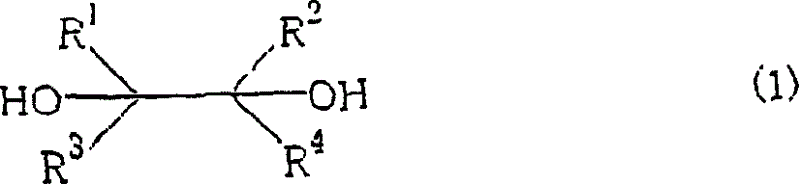Process for production of carbonyl compounds
一种羰基化合物、制造方法的技术,应用在碳基化合物制备、有机化合物的制备、氧化制备羰基化合物等方向,能够解决难以获得醛类、不能充分地满足工业上的需要、重金属废弃物处理麻烦等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] In a 100mL four-necked flask with a stirring device and a reflux cooling tube, drop into trans-3,3-dimethyl-2-(1,2-dihydroxy-2-methylpropyl)cyclopropanecarboxylic acid methyl ester (Content: 94.1% by weight) 0.2 g, 30 mL of acetonitrile, 0.1 g of triphenylbismuth, and 13.1 g of potassium carbonate. Methyl cyclopropanecarboxylate (content : 94.1% by weight) 10 ml of acetonitrile solution of 2 g, and 10 mL of acetonitrile solution in which 1.7 g of bromine was dissolved. Stir at this temperature for 30 minutes to allow it to react, then filter the insoluble components from the reaction solution, and wash with about 25 mL of acetonitrile to obtain a compound containing trans-3,3-dimethyl-2-formylcyclopropanecarboxylate The organic layer of methyl ester was 62.1 g.
[0056] Content of methyl trans-3,3-dimethyl-2-formylcyclopropanecarboxylate: 2.1% by weight.
[0057] Yield: 88%.
Embodiment 2
[0059] In a 100mL four-necked flask with a stirring device and a reflux cooling tube, drop into trans-3,3-dimethyl-2-(1,2-dihydroxy-2-methylpropyl)cyclopropanecarboxylic acid methyl ester (content: 94.1% by weight) 2.2 g, acetonitrile (3% by weight containing water) 30 mL, triphenylbismuth 0.05 g, succinimide 0.1 g, and potassium carbonate 5.3 g. 20 mL of an acetonitrile solution in which 1.9 g of bromine was dissolved was dropped therein at room temperature over 3.8 hours. Stir at this temperature for 30 minutes to allow it to react, then filter the insoluble components from the reaction solution, and wash with about 25 mL of acetonitrile to obtain a compound containing trans-3,3-dimethyl-2-formylcyclopropanecarboxylate 60.6 g of the organic layer of methyl ester.
[0060] Content of methyl trans-3,3-dimethyl-2-formylcyclopropanecarboxylate: 2.1% by weight.
[0061] Yield: 86%.
Embodiment 3
[0063]In a 100mL four-necked flask with a stirring device and a reflux cooling tube, drop into trans-3,3-dimethyl-2-(1,2-dihydroxy-2-methylpropyl)cyclopropanecarboxylic acid methyl ester (Content: 94% by weight) 0.22 g, acetonitrile (containing 3% by weight of water) 20 mL, succinimide group 0.1 g, triphenylbismuth 0.05 g, and potassium carbonate 5.3 g. Into it at an internal temperature of 40° C., 3 hours and 3.8 hours were added dropwise in parallel to dissolve trans-3,3-dimethyl-2-(1,2-dihydroxy-2-methylpropyl) ring 10 mL of an acetonitrile (3% by weight water) solution of 1.98 g of methyl propanecarboxylate (content: 94% by weight) and 20 mL of an acetonitrile (3% by weight water) solution in which 1.9 g of bromine was dissolved. Stir at this temperature for 30 minutes to allow it to react, then filter the insoluble components from the reaction solution, and wash with about 25 mL of acetonitrile to obtain a compound containing trans-3,3-dimethyl-2-formylcyclopropanecarboxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com