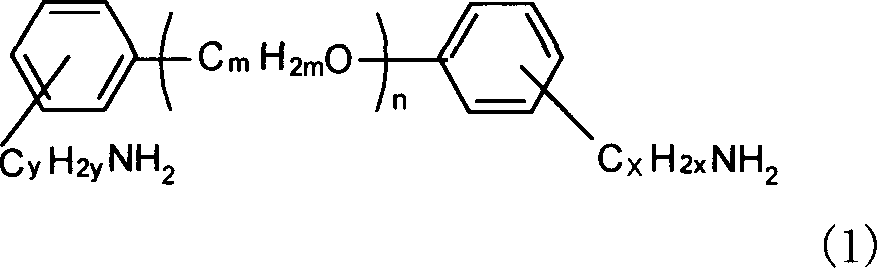
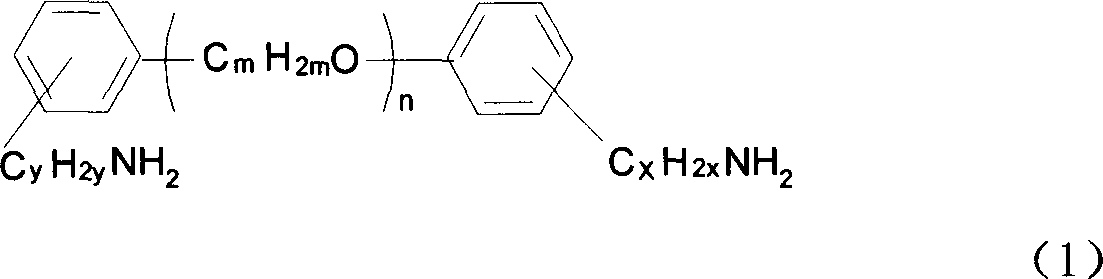
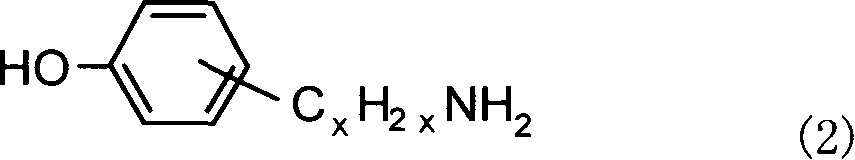Alpha, omega end amino polyether used as epoxy resin curing agent and method for preparing the same
A technology of epoxy resin curing and amino-terminated polyether, which is applied in the α field, can solve the problems of difficult curing, long curing time, unfavorable large-area construction application and wide use, and achieves a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: Add 0.05mol of polyethylene glycol and 0.6mL (0.01mol) of N,N-dimethylformamide (DMF) into a 100mL three-necked flask, heat up to 40°C with an oil bath while stirring, and then 8 mL (0.11 mol) of thionyl chloride was added dropwise within 10 min, and then the reaction was continued at 80° C. for 6 hours. SO produced during the reaction 2 , HCl gas is absorbed with lye. After the reaction solution was cooled, it was washed with saturated brine, the crude product was collected, dried with anhydrous potassium carbonate and adjusted to neutrality, and then the filtrate was collected by filtration to obtain chlorine-terminated polyethylene glycol.
[0015] In a 250ml three-necked flask, 0.1mol of hydroxybenzylamine, 0.4mol of NaOH and 0.84g of TBAB were mixed uniformly at room temperature, and then 0.05mol of chlorine-terminated polyethylene glycol was added. At the same time, the mixture was heated to 60° C. with an oil bath and stirred for 48 hours. After the...
Embodiment 2
[0016] Example 2: Add 0.05mol of polyethylene glycol and 0.15mL (0.0025mol) of N,N-dimethylformamide (DMF) into a 100mL three-necked flask, heat up to 40°C with an oil bath while stirring, and then 5.8 mL (0.08 mol) of thionyl chloride was added dropwise within 8 minutes, and then the reaction was continued at 60° C. for 30 hours. SO produced during the reaction 2 , HCl gas is absorbed with lye. After the reaction solution was cooled, it was washed with saturated brine, the crude product was collected, dried with anhydrous potassium carbonate and adjusted to neutrality, and then the filtrate was collected by filtration to obtain chlorine-terminated polyethylene glycol.
[0017] In a 250ml three-necked flask, 0.08mol of hydroxybenzylamine, 0.09mol of NaOH and 0.19g of TBAB were mixed uniformly at room temperature, and then 0.05mol of chlorine-terminated polyethylene glycol was added. Simultaneously, the mixture was heated to 50° C. with an oil bath and stirred for 4 hours. A...
Embodiment 3
[0018] Example 3: Add 0.05mol of polyethylene glycol and 1.5mL (0.025mol) of N,N-dimethylformamide (DMF) into a 100mL three-necked flask, heat up to 40°C with an oil bath while stirring, and then 14.5 mL (0.2 mol) of thionyl chloride was added dropwise within 15 min, and then the reaction was continued at 40° C. for 3 hours. SO produced during the reaction 2 , HCl gas is absorbed with lye. After the reaction solution was cooled, it was washed with saturated brine, the crude product was collected, dried with anhydrous potassium carbonate and adjusted to neutrality, and then the filtrate was collected by filtration to obtain chlorine-terminated polyethylene glycol.
[0019] In a 250ml three-necked flask, 0.15mol of hydroxybenzylamine, 0.6mol of NaOH and 1.9g of TBAB were uniformly mixed at room temperature, and then 0.05mol of chlorine-terminated polyethylene glycol was added. At the same time, the mixture was heated to 80° C. with an oil bath and stirred for 96 hours. After ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Peel strength | aaaaa | aaaaa |
| Adhesion | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com