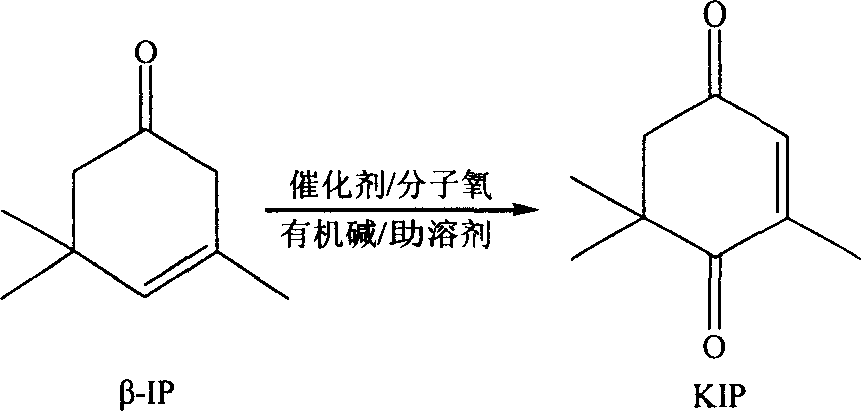
Process for preparing ketoisophorone
A technology for oxoisophorone and isophorone, which is applied in the field of preparation of oxoisophorone, can solve the problems of high process cost, easily destroyed catalyst, decreased reaction selectivity and the like, and achieves good reaction selectivity. , The effect of high reaction conversion rate and less catalyst dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Weigh 0.1g of catalyst (1#), dissolve it with 5ml of co-solvent DMF, and add it into a 250ml four-necked flask. Then add 50g of β-isophorone and 10ml of THF successively, and in a water bath at 80°C, feed oxygen while vigorously stirring. After 4 hours, the reaction is complete, the conversion rate is above 98%, and the selectivity reaches 88.5%.
Embodiment 2
[0033] Weigh 0.25g of the catalyst (2#), dissolve it with 5ml of co-solvent DMF and add it into a 250ml four-necked bottle. Then add 50g of β-isophorone and 10ml of pyridine successively, in a water bath at 40°C, feed oxygen, and stir vigorously at the same time, after 15 hours, the reaction is complete, the conversion rate is above 98%, and the selectivity reaches 95.5%.
Embodiment 3-10
[0035] Similar to embodiment 1, 2, respectively use the catalyst of different compositions, obtain following result (table one) after reaction finishes:
[0036] implement
example
BIP
(g)
catalyst
(g)
organic base
(ml)
Co-solvent
(ml)
temperature
(℃)
total time
(h)
Conversion rate
(%)
selectivity
(%)
3
50
3#0.10
Ethylenediamine 10
Methanol 5
80
8.5
92.5
55.6
4
50
4#0.10
Triethylamine 10
THF 5
80
7
94.5
72.5
5
50
5#0.10
Pyridine 10
DMF 5
80
4
98.5
85.6
6
50
6#0.10
Ethylenediamine 20
DMSO 5
80
4.5
98
88.1
7
50
7#0.25
Triethylamine 20
Methanol 5
40
14.5
98.8
93.2
8
50
8#0.25
Pyridine 20
THF 5
40
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com