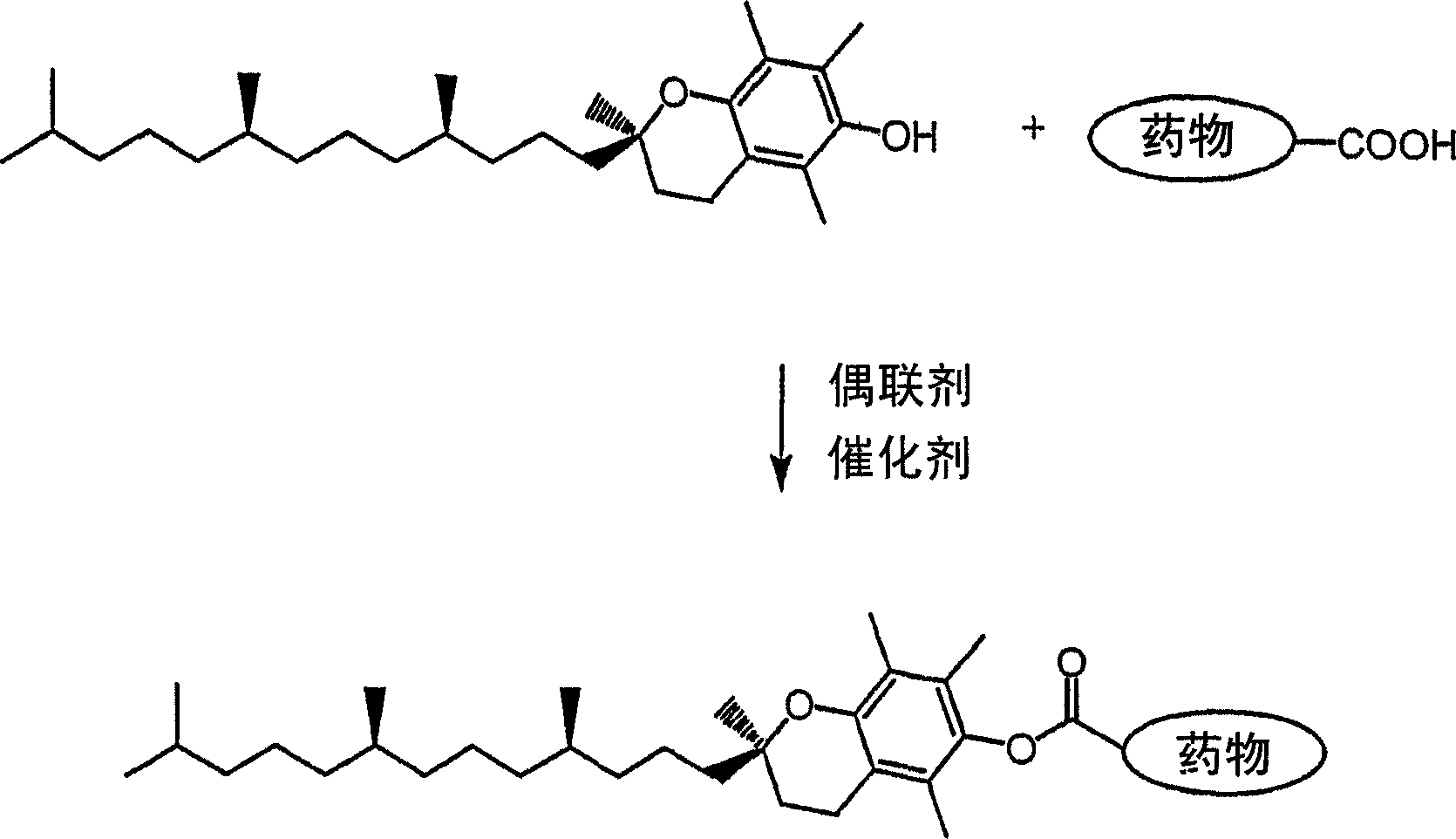
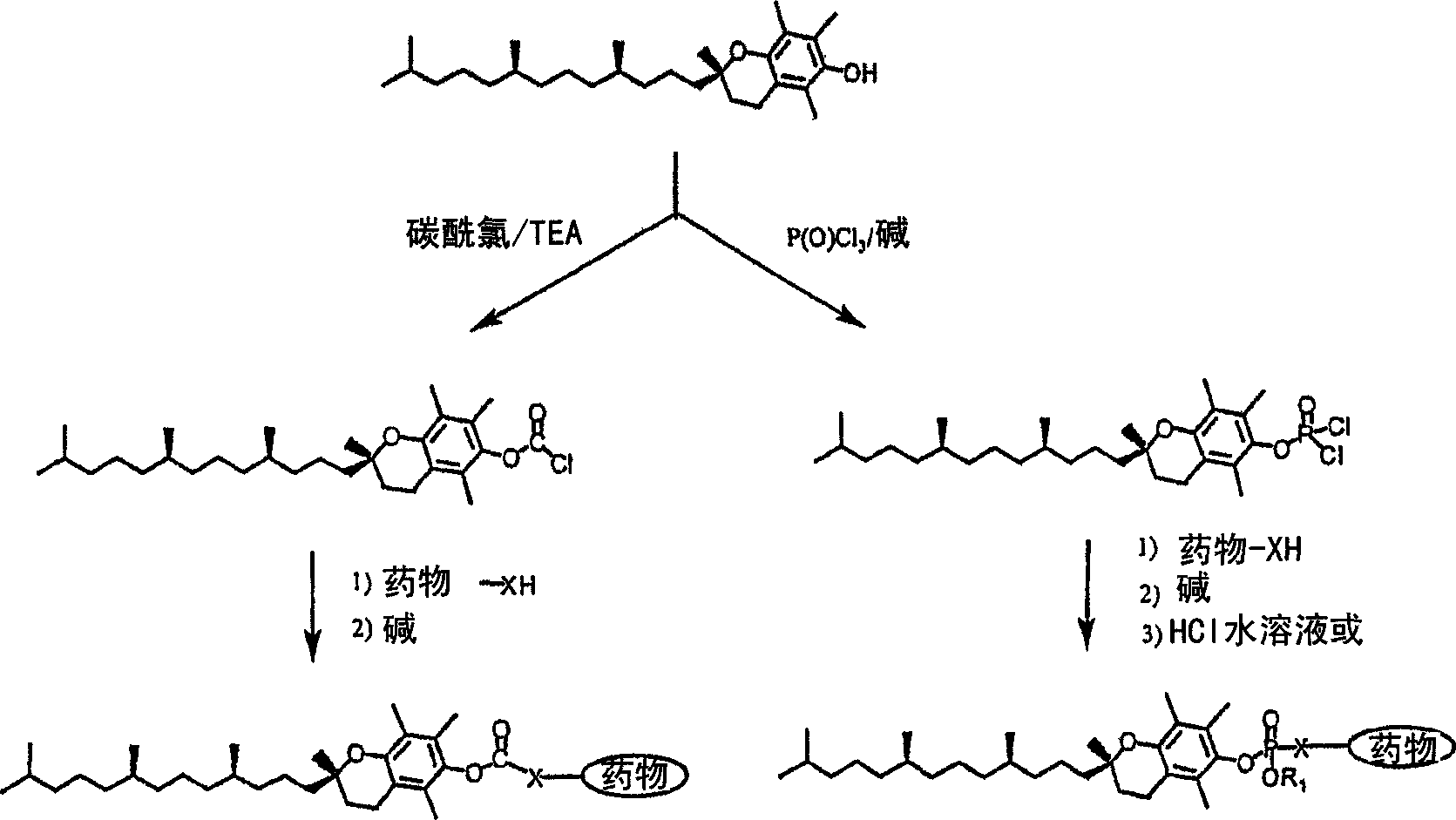
Tocopherol-modified therapeutic drug compounds
A drug compound, compound technology, applied in drug combination, sugar derivatives, organic chemistry, etc., can solve problems such as limited solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
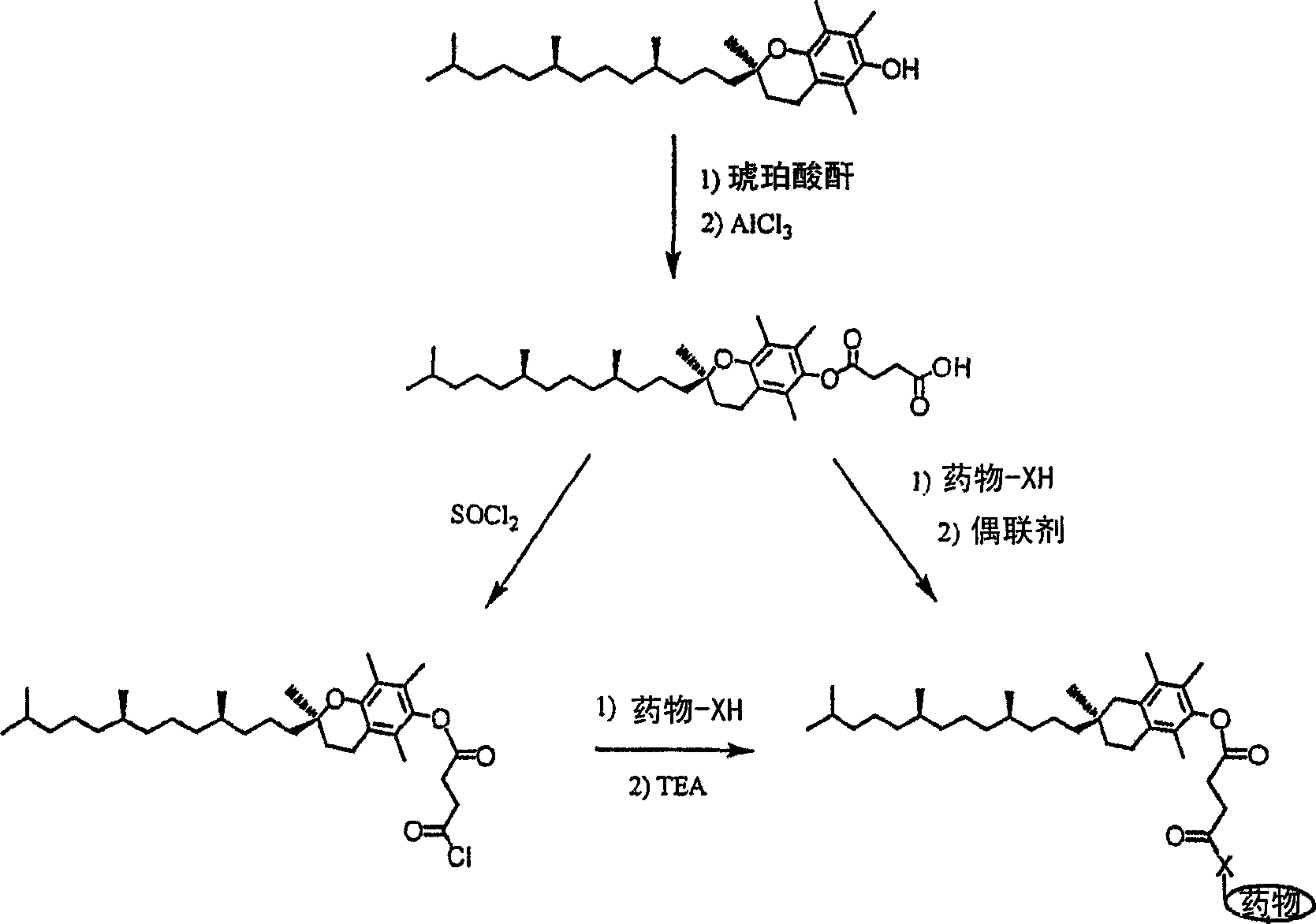
[0129] Figure 5 The preparation of tocopheryl succinate 10-hydroxycamptothecin and tocopheryl succinate 7-ethyl-10-hydroxycamptothecin (SN38) is described. Tocopheryl succinate is converted to the corresponding acid chloride, which is then reacted with 10-hydroxycamptothecin or 7-ethyl-10-hydroxycamptothecin (SN38). The preparation of tocopheryl succinate 10-hydroxycamptothecin and tocopheryl succinate 7-ethyl-10-hydroxycamptothecin is described in Examples 2 and 3, respectively.
[0130] Figure 6 The preparation of 10,20-bis(tocopheryl succinate) SN38 containing one therapeutic drug (SN38) moiety, two tocopherol moieties and two linking moieties (succinyl groups) is described. The preparation of 10,20-bis(tocopheryl succinate) SN38 is described in Example 4.
[0131] Suitable linking moieties may include oligomers or polymers such as peptides, polypeptides, proteins, mono-, di- or polysaccharides, oligomers of ethylene glycol, poly(ethylene glycol), poly(alkylene oxide) ...
Embodiment 14
[0145] Example 14 compares the solubility of two representative tocopherol-modified camptothecin compounds of the invention and camptothecin in several media.
[0146] Further aspects of the invention provide emulsion, microemulsion and micellar formulations containing the compounds of the invention. Also provided are methods for making emulsion, microemulsion, and micellar formulations.
[0147] As used herein, the term "emulsion" refers to two immiscible liquids, such as a colloidal dispersion of oil and water in droplet form, each droplet generally being between 0.1 and 3.0 microns in diameter, unless the dispersed and continuous phases are Index-matched, emulsions are usually opaque. The limited stability of such systems, generally limited by the application or relative reference system, can be enhanced by the addition of amphiphilic molecules or viscosity enhancers.
[0148] The term "microemulsion" refers to a thermodynamically stable isotropic clear dispersion of two ...
Embodiment 15
[0156] Example 15 describes therapeutic drug compounds containing tocopherol modifications (e.g., tocopheryl succinate docetaxel, tocopheryl succinate paclitaxel, tocopheryl succinate camptothecin, tocopheryl succinate 7-beta A representative emulsion of tocopheryl-10-hydroxycamptothecin and tocopheryl succinate 10-hydroxycamptothecin). Example 16 describes the in vitro cytotoxicity of representative tocopherol-modified therapeutic drug compounds (eg, tocopheryl succinate 7-ethyl-10-hydroxycamptothecin and tocopheryl succinate camptothecin).
[0157] Further aspects of the invention provide micellar formulations comprising a compound of the invention and an aqueous phase. Micelles are organized aggregates of one or more surfactants in solution. In one embodiment, the compound is present in the formulation in an amount of about 0.005-3.0% by weight, based on the total weight of the formulation. In one embodiment, the compound is present in the formulation in an amount of abou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com