(3-oxo-3, 4-dihydro-quinoxalin-2-yl-amino) -benzamide derivatives and related compound as glycogen phosphorylase inhibitors for the treatment of diabetes and obesity
A kind of technology of benzamide and compound, applied in the field of quinoxalinone
Inactive Publication Date: 2007-01-24
JANSSEN PHARMA NV
View PDF0 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Alternative approaches to inhibiting glycogenolysis to reduce HGP have not been fully explored
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0464] Enzyme Inhibition Assay:
[0465] compound
[0466] 30
[0467] G. Other implementations
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
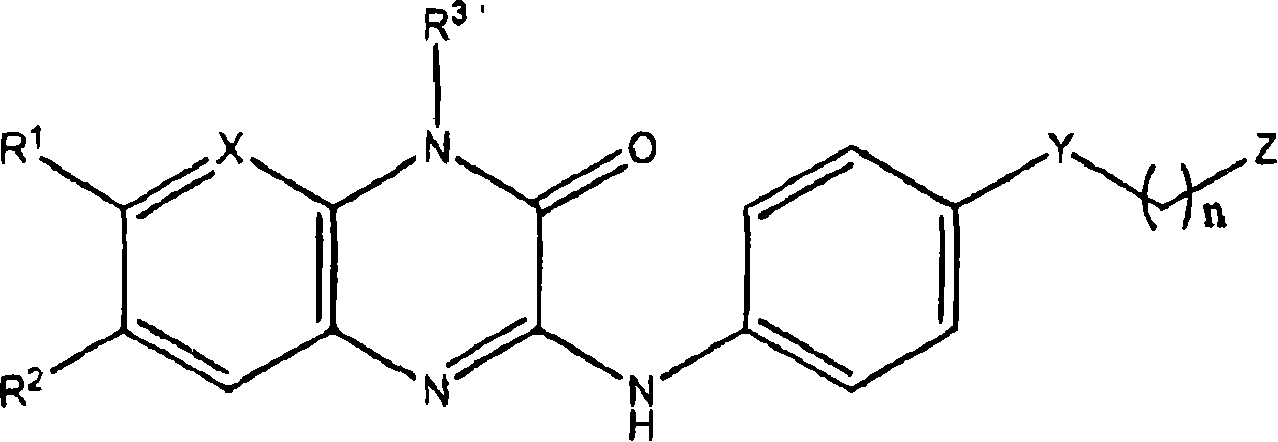
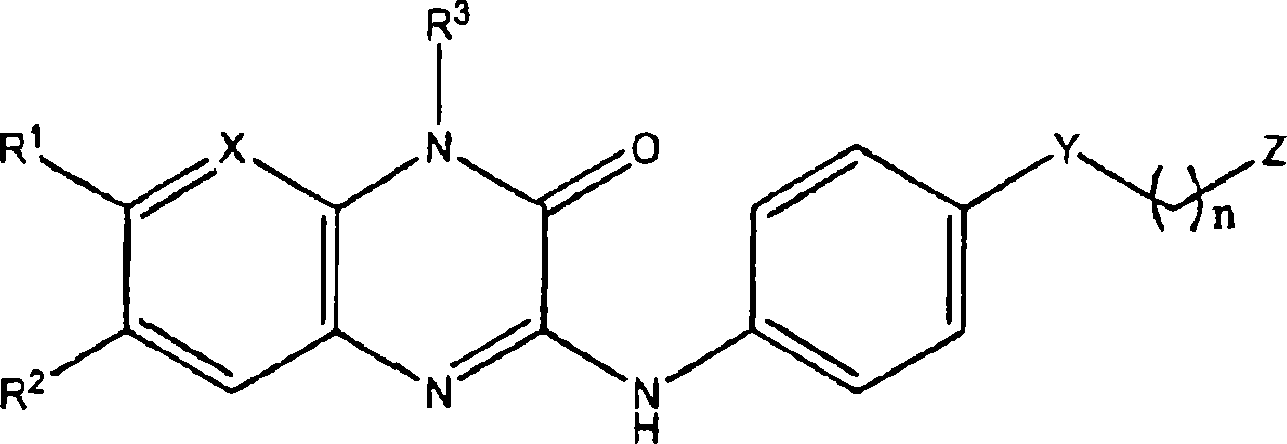
The invention features pharmaceutically active quinoxalinones of formula (1), compositions containing them, and methods of making and using theme: Formula (I) wherein R<1> is H, C 1-6 alkyl, or halo; R<2> is H or halo; R<3> is H, C 1-6 alkyl, X is N or CH; Y is a covalent bond, -NHCO- or -CONH-; Z is phenyl or a 5 or 6-membered heterocyclyl with between l and 2 heteroatoms independently selected from N, O, and S; and n is 0, 1 or 2; or a pharmaceutically acceptable salt, ester, amide, hydrate, or solvate thereof; as well as their use as glycogen phosphorylase inhibitors for the treatment of i diabetes and obesity.
Description
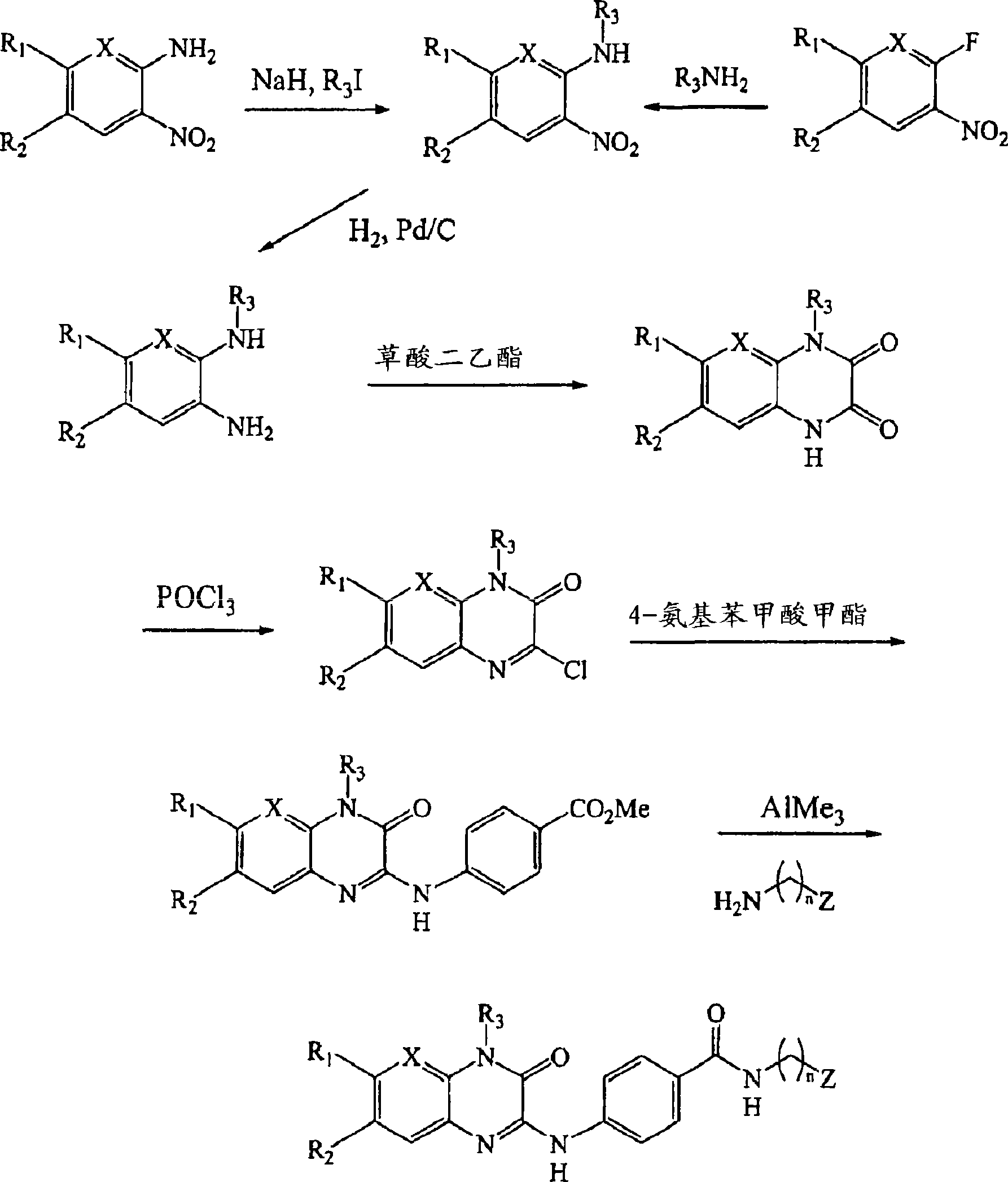
Background of the invention [0001] Non-insulin-dependent diabetes mellitus (NIDDM) is a polygenic disease characterized by defective insulin function in multiple target tissues including muscle, fat and liver. It is well established that normal glucose homeostasis requires accurate metabolic control in all insulin-responsive tissues; however the relative importance of each of these tissues to the pathogenesis of NIDDM is unclear. [0002] More specifically, maintenance of normal glucose homeostasis involves a highly coordinated series of events mediated by a variety of cellular proteins that catalyze 1) the transport and phosphorylation of glucose, 2) the phosphorylation of The combination of glucose monosaccharides into glycogen, and 3) glycogen hydrolysis and hepatic glucose production. Each of these processes represents a site of possible therapeutic intervention. Several pathways regulating hepatic glucose production (HGP) are involved in the regulation of circulating bl...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/498A61K31/4985C07D413/12C07D403/12C07D417/12C07D409/12C07D407/12C07D471/04C07D241/44A61P3/10A61K31/5377C07D413/02C07D487/02
CPCC07D401/12C07D471/04C07D403/12C07D409/12C07D405/12C07D417/12C07D413/12C07D241/44A61P3/00A61P3/04A61P3/10
Inventor M·P·比弗斯J·杜达什Y·张
Owner JANSSEN PHARMA NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com