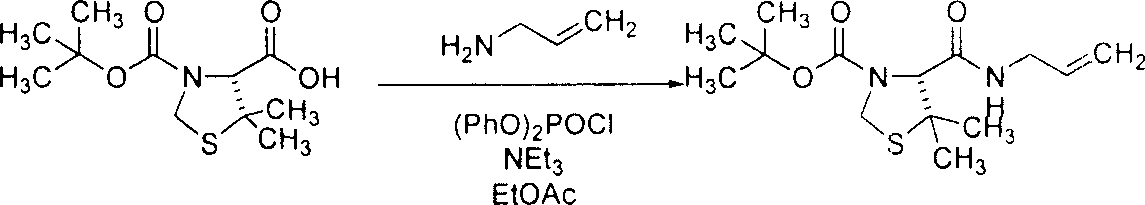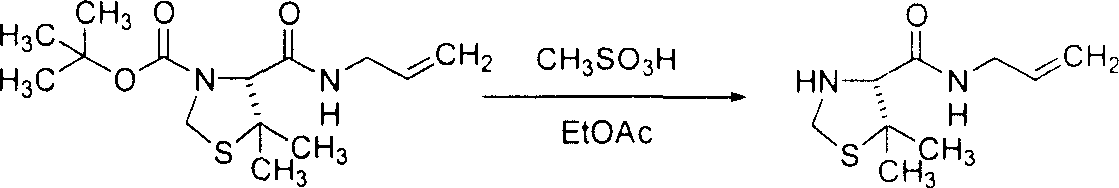Compositions comprising HIV protease inhibitor and cytochrome P450 enzyme activity inhibitor
A technology of inhibiting cells and compositions, applied in the direction of organic active ingredients, active ingredients of heterocyclic compounds, medical preparations containing active ingredients, etc., can solve the problems of ineffectiveness and ineffectiveness of diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
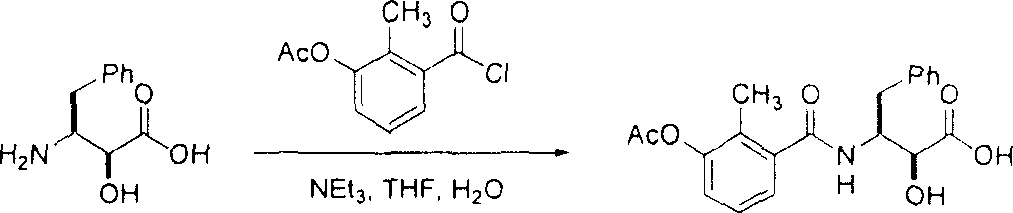
[0132] Example 1: (4R)-N-allyl-3-{(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-phenyl Butyryl}-5,5-dimethyl-1,3-thiazolidine-4-carboxamide (Compound A) administered alone or with 10-hydroxy-2-methyl-5-(1-methylethyl )-1-[2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-2,4,7,12- The combined administration of tetraazatridecane-13-acid, 5-thiazolyl methyl ester, [5S-(5R*, 8R*, 10R*, 11R*)] (ritonavir).
[0133] Under the feeding regimen according to the schedule appearing in Table 1, a single 100mg, 300mg, 800mg, 1000mg, 1500mg, 2000mg or 3000mg spray-dried composition was administered to a total of 30 human subjects, which contained 90wt% The amorphous compound A and 10wt% of hydroxypropyl methylcellulose acetate succinate (HPMCAS). In addition, a total of 12 human subjects were administered, and 400 mg or 800 mg of spray-dried composition containing 90% by weight of amorphous compound A and 10% by weight of hydroxypropyl methylcellulose acetate succinat...
Embodiment 2
[0136] Example 2: 4,4-Difluoro-1-{(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-phenylbutyryl} -3,3-Dimethyl-N-(2,2,2-trifluoroethyl)-L-prolineamide (Compound B) administered alone or with 10-hydroxy-2-methyl-5-( 1-methylethyl)-1-[2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-2, 4,7,12-Tetraazatridecane-13-acid, 5-thiazolyl methyl ester [5S-(5R*, 8R*, 10R*, 11R*)] (ritonavir) combined administration .
[0137] A single 300mg, 900mg, 1800mg or 3600mg spray-dried composition was administered to a total of 21 human subjects under fasting conditions, which contained 90wt% of amorphous compound B and 10wt% of hydroxypropyl methylcellulose acetate Hydroxypropylmethyl cellulose acetate succinate (HPMCAS). In addition, a total of 12 human subjects were given 300 mg or 450 mg of a spray-dried composition containing 90% by weight of amorphous compound B and 10% by weight of hydroxypropyl methylcellulose acetate succinate (HPMCAS), and ritona The combination of v...
Embodiment 3
[0143] Example 3: Preparation of (4R)-4-allylcarbamoyl-5,5-dimethyl-thiazolidine-3-carboxylic acid tert-butyl ester
[0144]
[0145] (4R)-5,5-dimethyl-thiazolidine-3,4-dicarboxylic acid 3-tert-butyl ester (according to Ikunaka, M. et al., Tetrahedron Asymm. 2002, 13, 1201; Mimoto , T. et al., J. Med. Chem. 1999, 42, 1789; and Mimoto, T. et al., European Patent Application 0574135A1 (1993) method to prepare, 250g; 0.957mol) was added to an argon flush And dissolved in EtOAc (1.25L). Cool the solution to 2°C, then add (PhO) 2 POCl (208 mL; 1.00 mol) was added to the solution all at once. NEt 3 (280 mL; 2.01 mol) was added dropwise to the solution via a funnel, and then the resulting suspension was stirred at 0°C. Seven minutes later, allylamine (75.4 mL; 1.00 mol) was added dropwise. The ice bath was removed, and the suspension was warmed to room temperature. After half an hour, 1N HCl (750 mL; 0.750 mol) was added. The mixture was transferred to a 4-L separatory funnel and rinse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com