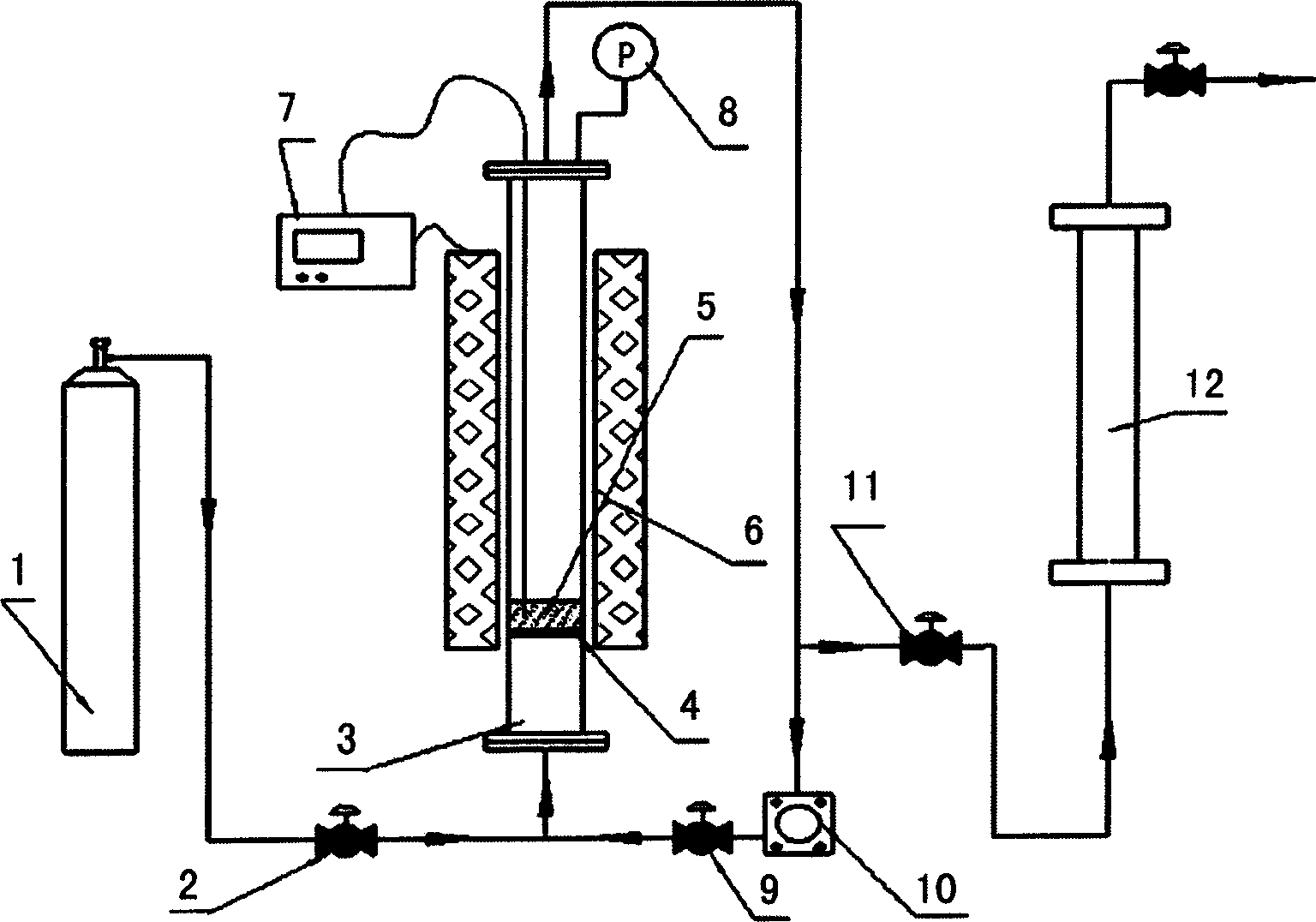
Technique for synthesizing graphite fluoride and carbon fluoride by using nitrogen trifluoride as fluridizer
A technology of ink, carbon fluoride, and nitrogen trifluoride, which is applied in the field of synthetic fluorinated graphite and carbon fluoride, can solve the problems of high product cost, poor safety, and short service life of equipment, and achieve low production cost and high safety. High efficiency and long service life of the equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
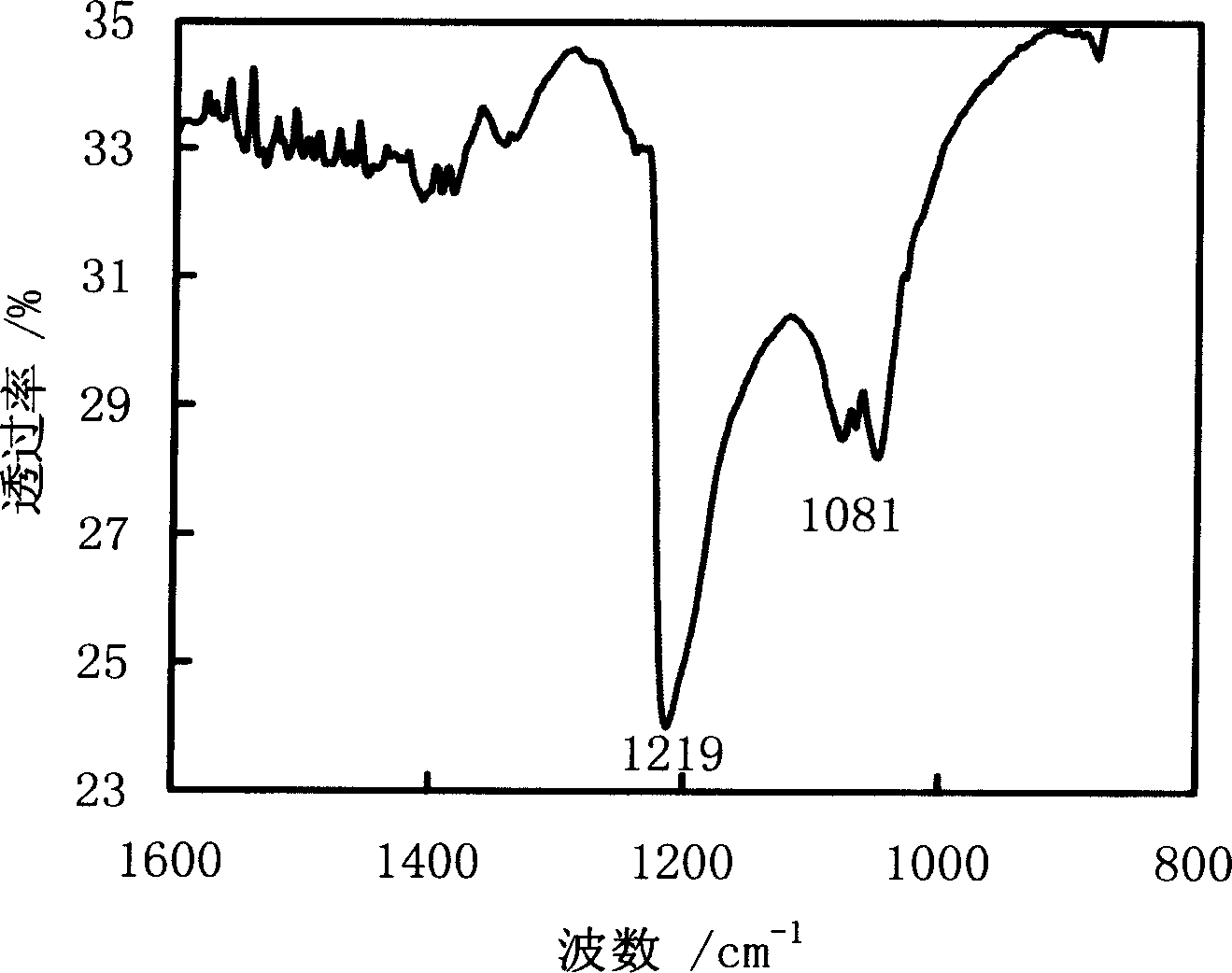
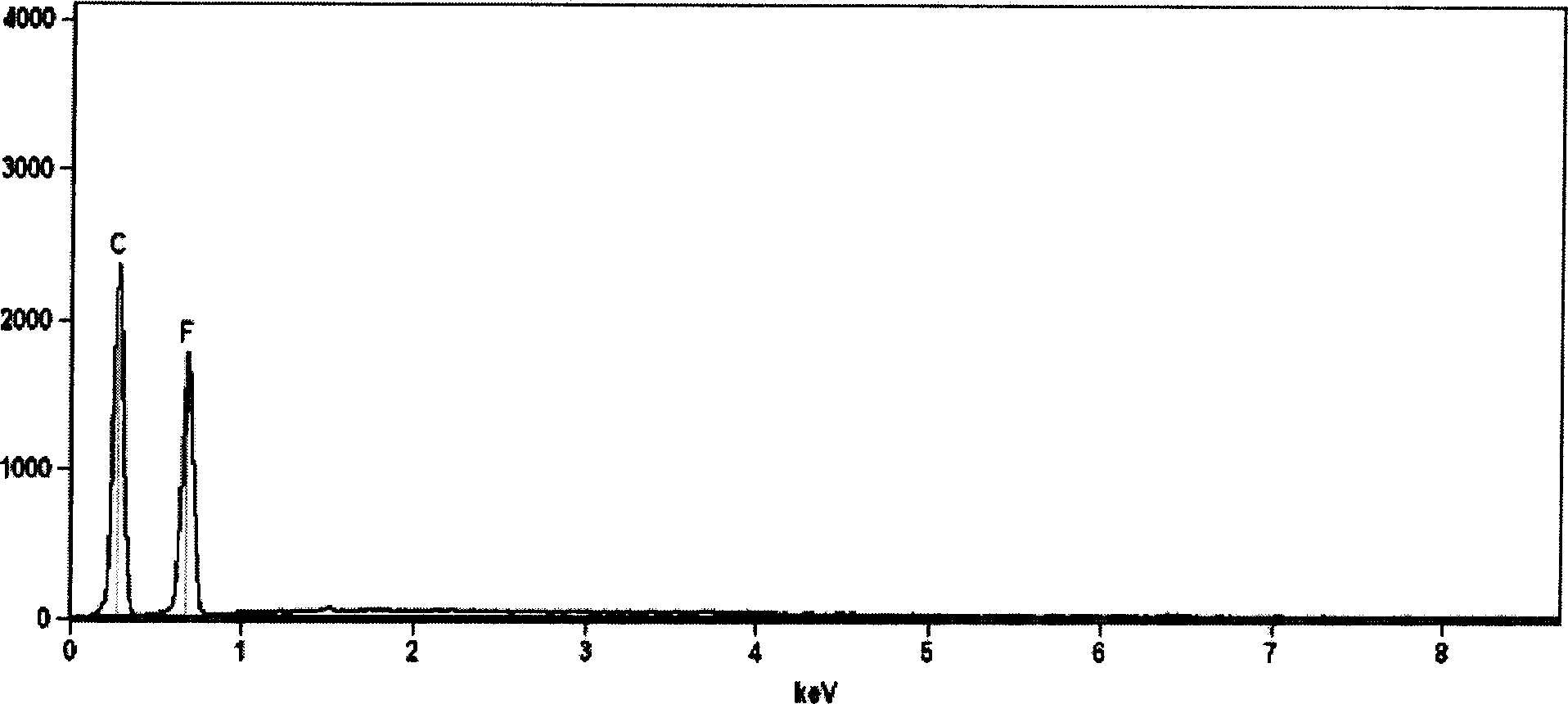
[0076] Put the dried graphite powder into the reactor, pass pure NF 3 Gas, the pressure reaches 150KPa. Raise the temperature, maintain the reaction temperature at 450° C., and react for 30 hours. The product was taken out after cooling, the fluorocarbon ratio of the product was 0.48, and the yield was 92%. The infrared absorption spectrum and elemental analysis EDS spectrum of the product are as follows figure 2 and image 3 shown.
Embodiment 2
[0078] Put the dried graphite powder into the reactor, pass pure NF 3 Gas, the pressure reaches 150KPa. Raise the temperature, maintain the reaction temperature at 540°C, and react for 15 hours. The product was taken out after cooling, the fluorocarbon ratio of the product was 0.95, and the yield was 87%. The infrared absorption spectrum and elemental analysis EDS spectrum of the product are as follows Figure 4 and Figure 5 shown.
Embodiment 3
[0080] Put the dried graphite powder into the reactor, pass pure NF 3 Gas, the pressure reaches 100KPa. Raise the temperature, maintain the reaction temperature at 640°C, and react for 4 hours. The product was taken out after cooling, the fluorocarbon ratio of the product was 1.12, and the yield was 55%. The infrared absorption spectrum and elemental analysis EDS spectrum of the product are as follows Figure 6 and Figure 7 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com