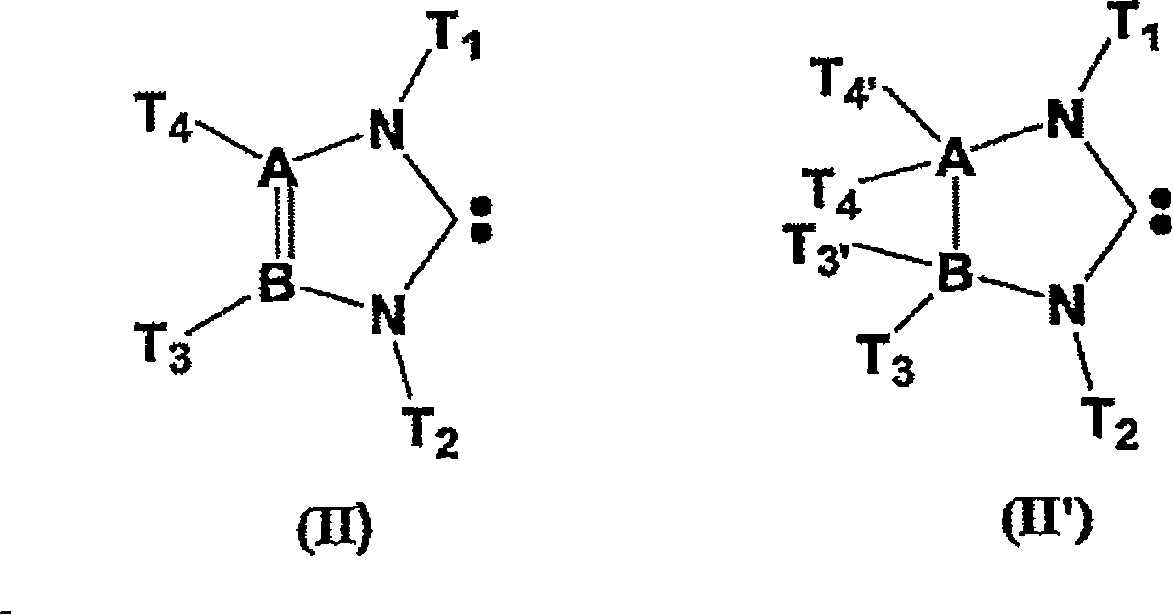
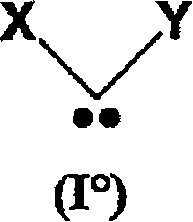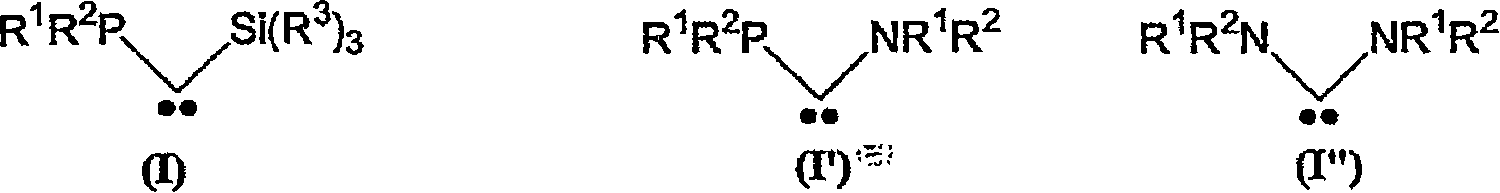Method for preparing polyorganosiloxane (pos) by ring(s)-opening polymerisation and/or pos redistribution in the presence of carbene(s) and pos compounds produced by said method
A polyorganosiloxane, redistribution technology, applied in the composition of POS and carbene catalysts, the ring-opening and/or redistribution polymerization of POScy to prepare POS field, can solve the problem of not being able to obtain high molecular weight, and achieve excellent Stability, simple operation, simple synthesis effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0245] 10 grams of octamethylcyclotetrasiloxane (D4) and 100 mg of hexamethylsiloxane (M2) were mixed in a 30 ml flask. 100 mg of imidazolium tetrafluoroborate 1 (0.34 mmol) and 39 mg of t-BuOK (0.34 mmol) were placed in a weighing tube. 0.5 mL of anhydrous THF was added, and the resulting suspension was quickly added to the flask containing M2 and D4.
[0246] The reaction mixture was stirred at room temperature for 24 hours. It slowly became viscous and silicon NMR analysis showed that 95% of the D4 was polymerized in the form of polysiloxane chains.
Embodiment 2
[0248] 10 grams of octamethylcyclotetrasiloxane (D4) and 100 mg of hexamethylsiloxane (M2) were mixed in a 30 ml flask. 100 mg of imidazolium tetrafluoroborate 1 (0.34 mmol) and 39 mg of t-BuOK (0.34 mmol) were placed in a weighing tube. 0.5 mL of anhydrous THF was added, and the resulting suspension was quickly added to the flask containing M2 and D4.
[0249] The reaction mixture was stirred at 60°C for 2 hours. It slowly became viscous and silicon NMR analysis showed that 95% of the D4 was polymerized in the form of polysiloxane chains.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com