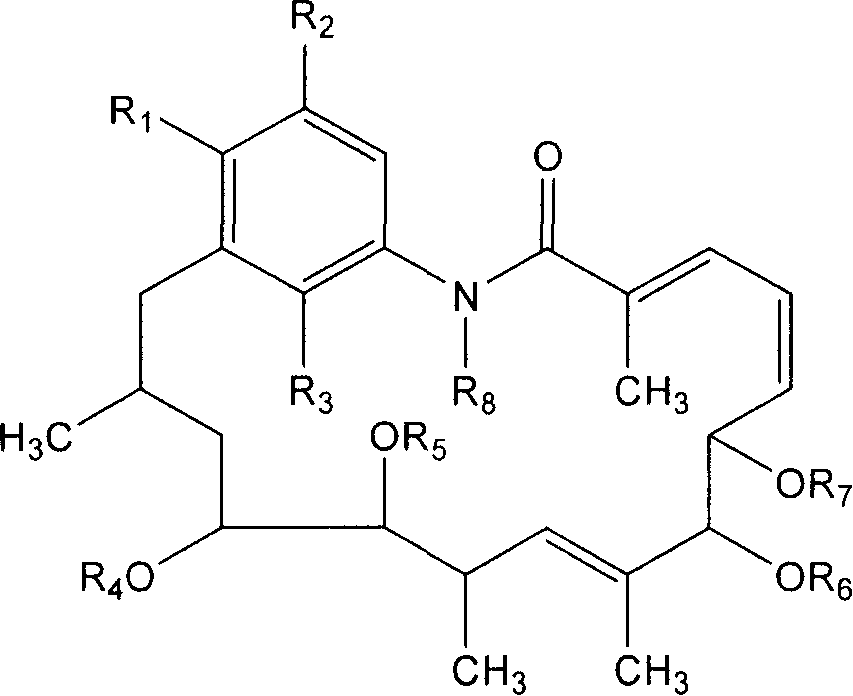
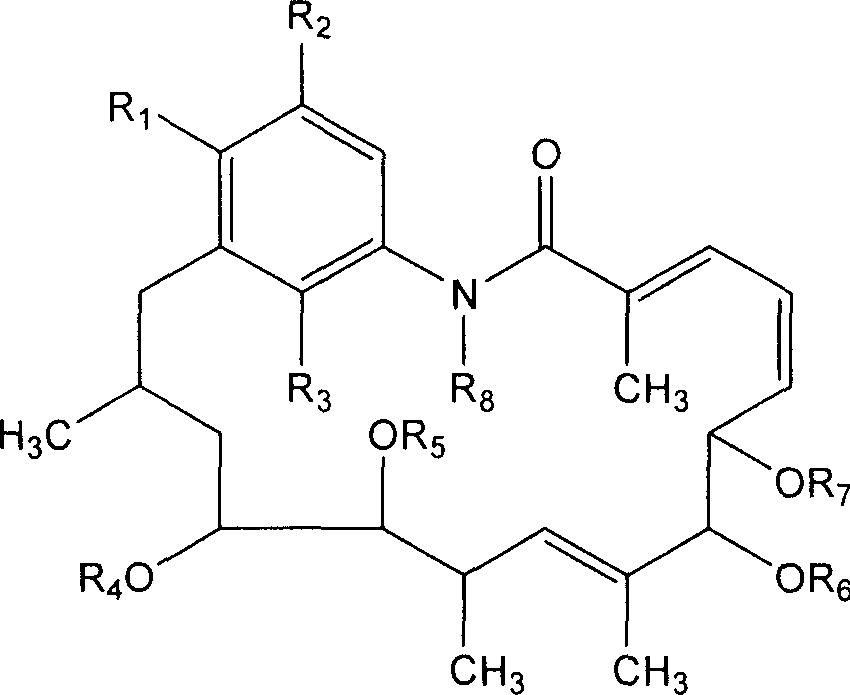
Aromatic reductive derivative of geldanamycin benzoquinone, preparation method and use thereof
A technique for geldanamycin and aromatization, which is applied in the fields of aromatized reduction derivatives of geldanamycin-like benzoquinones and their preparation and application, and can solve problems such as the reduction of benzoquinones that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Fermentative production and separation and purification of embodiment 1 compound 1
[0087] Fermentation production
[0088] Toxin-producing bacteria The toxin-producing bacteria used to ferment and produce Compound 1 in this example was isolated from soil samples collected from Xishuangbanna and identified as Streptomyces pseudoverticillus strain YN17707 CGMCC No.1452 through taxonomic studies.
[0089] Fermentative culture of toxin-producing bacteria According to the conventional method of cultivating microorganisms, take an appropriate amount of Streptomyces pseudoverticillus YN17707 CGMCC No.1452 strain, inoculate it into an eggplant-shaped bottle on Gaoshi Synthetic No. 1 agar solid slant medium, and cultivate it at 28 degrees Celsius Activation culture in the box for 7 days. Get the eggplant-shaped bottle slope that has been activated and cultivated for 7 days, add 15 milliliters of sterile water, scrape off the spores with an inoculation needle, make a spore sus...
Embodiment 2
[0097] The separation and purification of embodiment 2 compound 2
[0098] Get 30 grams of the chloroform extract obtained in the above-mentioned Example 1, dissolve it with an appropriate amount of chloroform, add 50 grams of silica gel G (200-300 mesh) produced by Qingdao Ocean Chemical Factory to mix the sample, add to the silica gel that is equipped with 300 grams of thin-layer chromatography. 60H (Qingdao Haiyang Chemical Factory) glass decompression column, carry out decompression column chromatography, use chloroform-methanol system (100:0→80:20) gradient elution, every 500ml is a fraction, and several fractions are obtained. share. According to the results of TLC detection and activity test, the corresponding fractions were combined to obtain the active component Fr-4 containing compound 2 (the elution fraction of chloroform-methanol 80:20). Dissolve the active component Fr-4 in an appropriate amount of chloroform, add an appropriate amount of silica gel G (200-300 me...
Embodiment 3
[0104] Example 3 Cell Cycle Inhibitory Activity Test of Compound 1 and Compound 2
[0105] Experimental samples and experimental methods
[0106] Preparation of test sample solution The test samples are pure compound 1 and compound 2 separated and purified in the above-mentioned examples 1 and 2. Accurately weigh an appropriate amount of sample, and use methanol to make a solution of the required concentration for testing the activity.
[0107] The subculture activity test of cell lines and cells adopts the temperature-sensitive mouse breast cancer tsFT210 cell line, and the cells are relayed in RPMI-1640 medium containing 10% FBS in a cell incubator with 5% carbon dioxide at 32 degrees Celsius subculture.
[0108] Flow Cytometry Activity Assay Method
[0109] asynchronous culture test
[0110] Take the tsFT210 cells in the logarithmic growth phase, and use fresh RPMI-1640 medium to prepare a density of 2×10 per ml 5 The cell suspension of each cell was inoculated in a 24...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com