Process for preparing P-tertiary butyl phenyl acetic ester organic compounds
A technology for ethyl tert-butyl phenylacetate and organic compounds, which is applied in the field of organic chemical compound preparation, can solve problems such as production and operation hazards, and achieve the effects of simple process and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
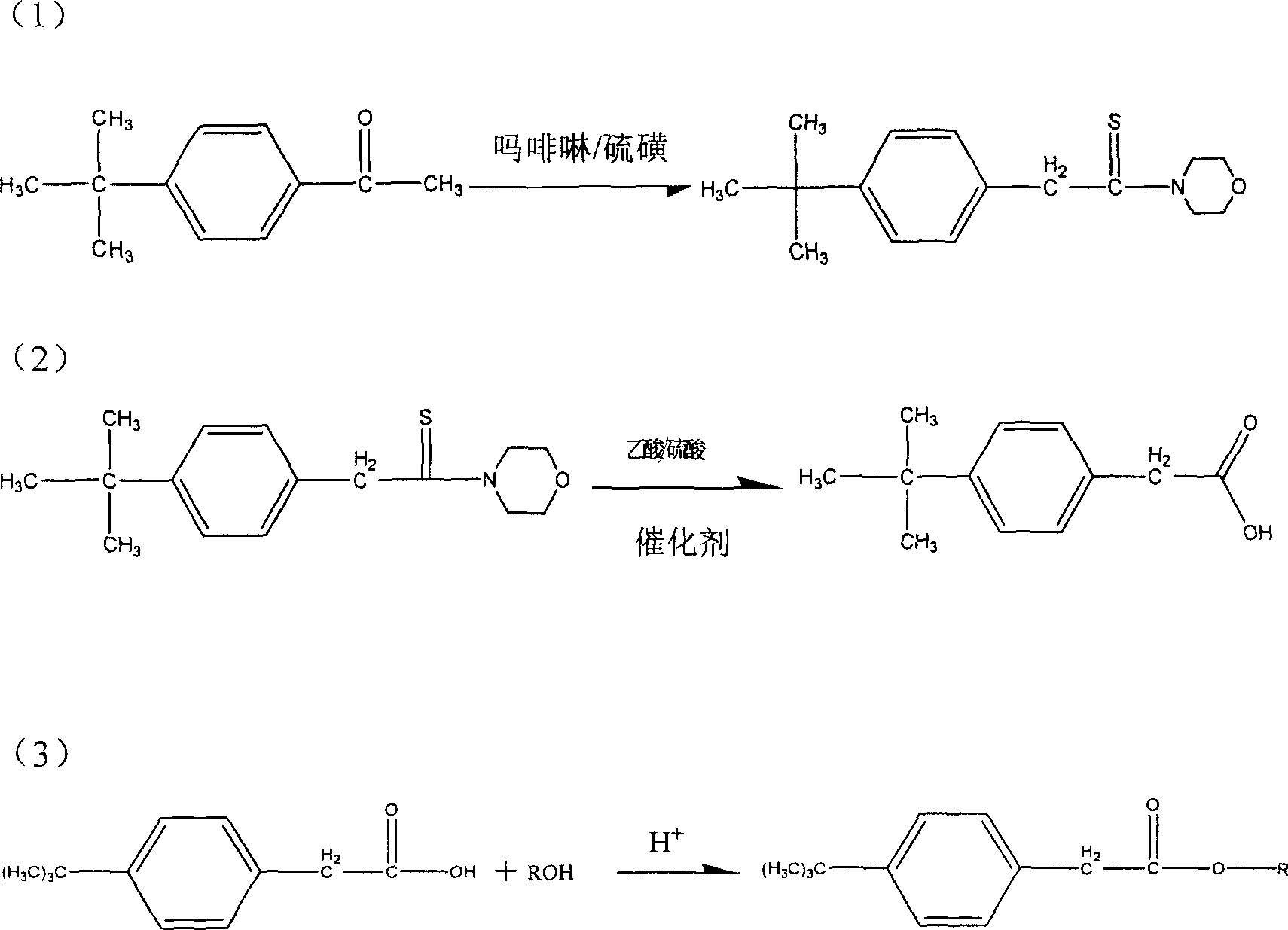
[0014] (1), prepare p-tert-butylphenylacetic acid:
[0015] Add 200g p-tert-butylacetophenone and 216g morpholine in a 500ml three-necked flask equipped with a stirrer and a reflux condenser, stir and mix, then add 92g sulfur, and heat the reaction under constant stirring; the temperature gradually rises from 110°C to The reaction temperature is 210°C; it begins to boil at 110°C, and reflux begins at 120°C; the reaction time is 12 hours; then it is cooled to 80°C, and the reaction solution is poured into 500ml of absolute ethanol to obtain yellow precipitated thio-tert-butylmorpholine Amide; put 100g of the yellow precipitate in 150ml of glacial acetic acid and 25ml of 98% sulfuric acid solution for acidification reaction, the reaction temperature is 114-118°C, and the acidification time is 3 hours; then pour the above-mentioned acidified reaction solution into Cool in cold water for 10 hours to obtain the crude product p-tert-butylphenylacetic acid; then recrystallize the cru...
Embodiment 2
[0020] This embodiment is the preparation method of p-tert-butylphenylacetic acid ethyl ester, and its preparation method is exactly the same as above-mentioned embodiment 1 p-tert-butylphenylacetic acid methyl ester.
[0021] (1), prepare p-tert-butylphenylacetic acid: its method is identical with above-mentioned embodiment 1.
[0022] (2), preparation of ethyl p-tert-butylphenylacetate: identical with the method of above-mentioned embodiment 1. The difference is:
[0023] (a) The consumption of raw material is different, adopts p-tert-butylphenylacetic acid 25g, dehydrated alcohol 100ml, sulfuric acid 20ml (98%); (b) when reaction temperature reaches 80 ℃, make it reflux heating 4 hours. The following process steps are exactly the same. Finally, colorless to pale yellow ethyl p-tert-butylphenylacetate was obtained.
[0024] The product has been tested to be 98% pure.
Embodiment 3
[0026] This embodiment is the preparation method of p-tert-butylphenylacetic acid n-butyl ester, and its preparation method is exactly the same as the above-mentioned embodiment 1 p-tert-butylphenylacetic acid methyl ester.
[0027] (1), prepare p-tert-butylphenylacetic acid: its method is identical with above-mentioned embodiment 1.
[0028] (2), preparation of n-butyl p-tert-butylphenylacetic acid: identical with the method of above-mentioned embodiment 1. The difference is: (a) the consumption of raw materials is different, adopt 25g of p-tert-butylphenylacetic acid, 100ml of n-butanol, 20ml of sulfuric acid (98%); . The following process steps are exactly the same. Finally, colorless to pale yellow n-butyl p-tert-butylphenylacetate was obtained.
[0029] The product has been tested to be 98% pure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com