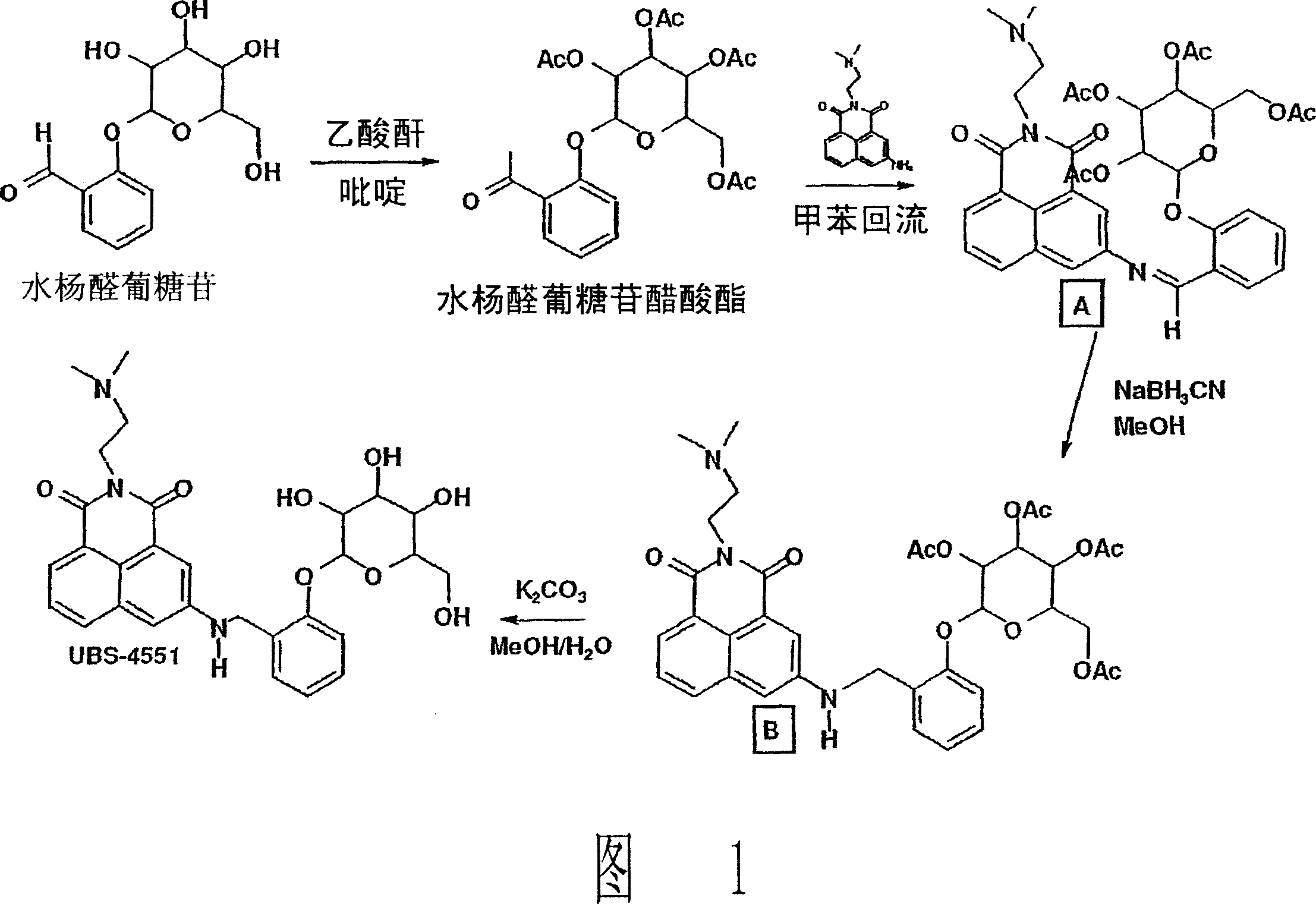
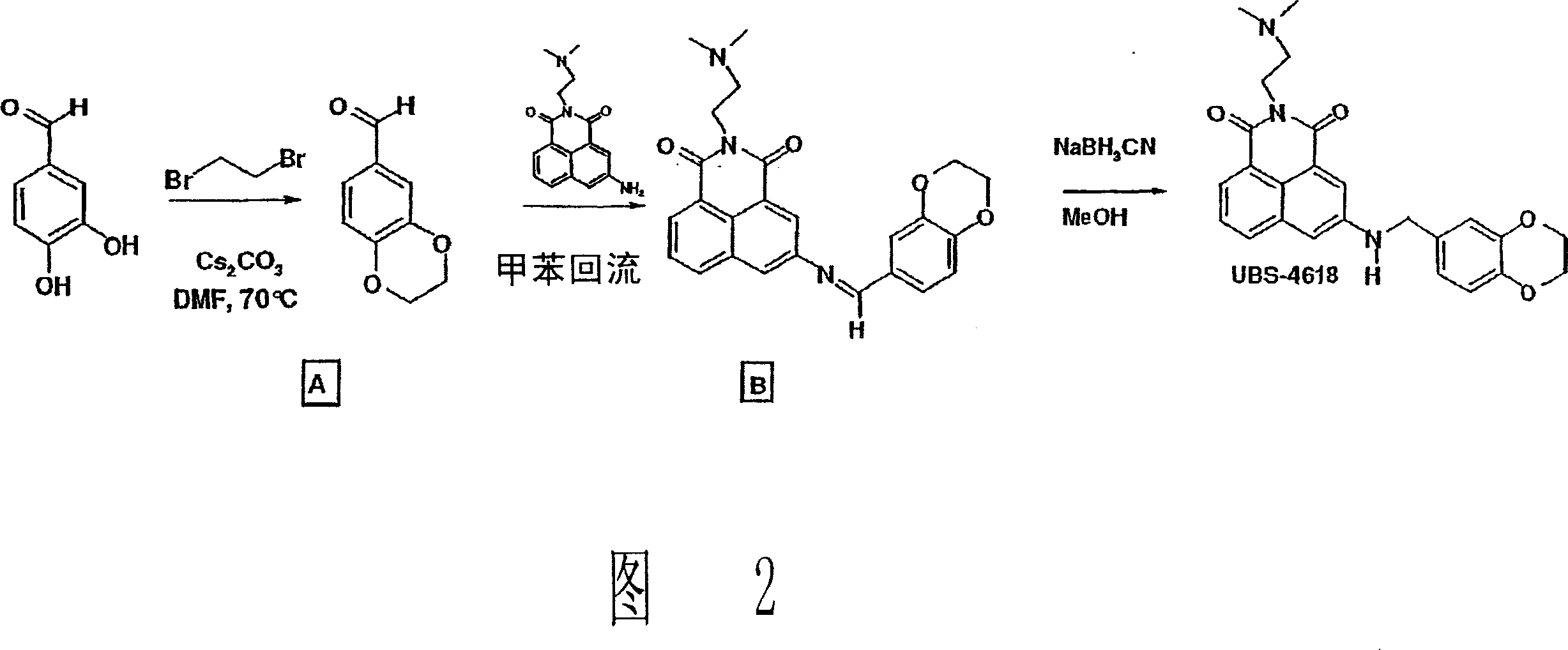
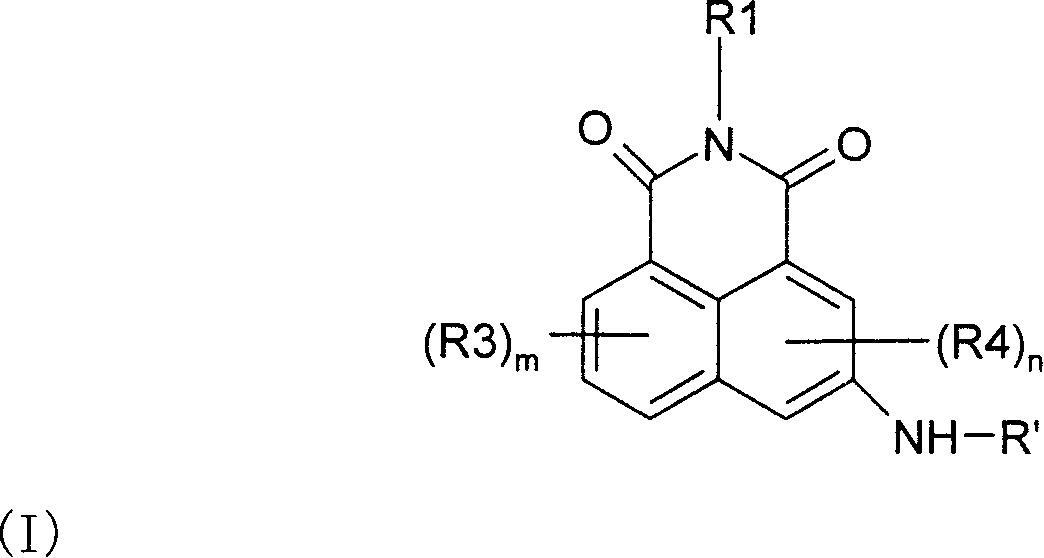
Naphthalimide derivatives for the treatment of cancer
A technology of naphthalimide and derivatives, applied in the field of naphthalimide derivatives for treating cancer, can solve the problems of low cytotoxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0075] -n=0, and / or
[0076] -m=0, and / or
[0077] -m=2, two substituents R 3 Adjacent and together with the carbon atom to which they are attached form a phenyl group, and / or
[0078] -R 1 It is an alkylene group with 1 to 3 carbon atoms, and is selected from the group consisting of dimethylamino, diethylamino, pyrrolidinyl, piperidino, N-methylpiperazinyl, morpholino and ureylene Is connected to the nitrogen-containing group, more preferably, R 1 Is dimethylene linked to dimethylamino or diethylamino, and / or
[0079] -R’ is selected from: C 2-7 Alkylcarbonyl, amino-carbonyl, thioaminocarbonyl, alkylaminocarbonyl, alkylthioaminocarbonyl, alkylthiocarbonyl, and poly(aminoalkyl), where the number of aminoalkyl repeating units ranges from 2 to about 5 In the range.
[0080] In the second aspect, the present invention provides a class of substituted naphthalimide (isoquinolindione) derivatives represented by general formula (II), and / or pharmaceutically acceptable salts and / or solvat...
Embodiment 1
[0144] Example 1-Preparation of 2-chloro-N-[({2-[2-(dimethylamino)ethyl]-1,3-dioxo-2,3-dihydro-1H-benzo[de ]Isoquinolin-5-yl}amino)carbonyl]acetamide
[0145] Under nitrogen, 100 mg of aminonafetil was dissolved in 2 mL of acetonitrile, and then a solution of 95 mg of 2-chloroacetyl isocyanate (2 equivalents) in 2.5 mL of acetonitrile was carefully added. The reaction was maintained at room temperature for 4 hours. Then, acetonitrile was evaporated under reduced pressure, and the residue was subjected to flash chromatography (SiO 2 , Eluent: CH 2 Cl 2 / MeOH 95:5), thus obtaining 25.5 mg (yield: 18%) of the desired product:
[0146]
[0147] Under 300MHz, in DMSO, it is characterized by proton nuclear magnetic resonance (hereinafter denoted as 1 H NMR), the results are as follows: 11.15 (H-17, bs); 10.30 (H-19, s); 8.57 (H-2, d, J=2.1); 8.53 (H-4, d, J=2.1) ; 8.34 (H-8, s); 8.32 (H-6, s); 7.79 (H-7, t, J = 7.8); 4.17 (H-13, t, J = 6.8), 3.76 (H-21 , S); 2.61 (H-14, t, J=6.6) and ...
Embodiment 2
[0148] Example 2-Preparation of 2,2,2-Trichloro-N-[({2-[2-(dimethylamino)ethyl]-1,3-dioxo-2,3-dihydro-1H -Benzo[de]isoquinolin-5-yl}amino)carbonyl]acetamide
[0149]
[0150] Under nitrogen, 700 mg of aminonafetil was dissolved in 14 mL of acetonitrile. Carefully add a solution of 932 mg trichloroacetyl isocyanate (2 equivalents) in 14 mL acetonitrile. The reaction was maintained at room temperature for 4.5 hours. Then acetonitrile was evaporated under reduced pressure, and the residue was subjected to flash chromatography (SiO 2 , Eluent: CH 2 Cl 2 / MeOH 97:3), thus 540.5mg (yield: 46%) of the desired product is obtained, which is characterized by:
[0151] - 1 H NMR (300MHz, DMSO) is as follows: 11.18 (H-17 and H-19, bs); 8.76 (H-2, bs); 8.75 (H-4, bs); 8.43 (H-8, d, J= 6.6); 8.41 (H-6, d, J=6.0); 7.85 (H-7, t, J=7.5); 4.20 (H-13, t, J=6.6), 2.69 (H-14, t, J=6.3) and 2.35 (H-15 and H-16, s) (the same number of atoms as in Example 1), and
[0152] -300MHz, carbon nuclear magnet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com