A pharmaceutical formulation and preparation process thereof
A technology for pharmaceutical preparations and excipients, applied in the field of recombinant virus preparations and their preparation, can solve the problems of affecting the cost of drugs, affecting the safety and effectiveness of drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
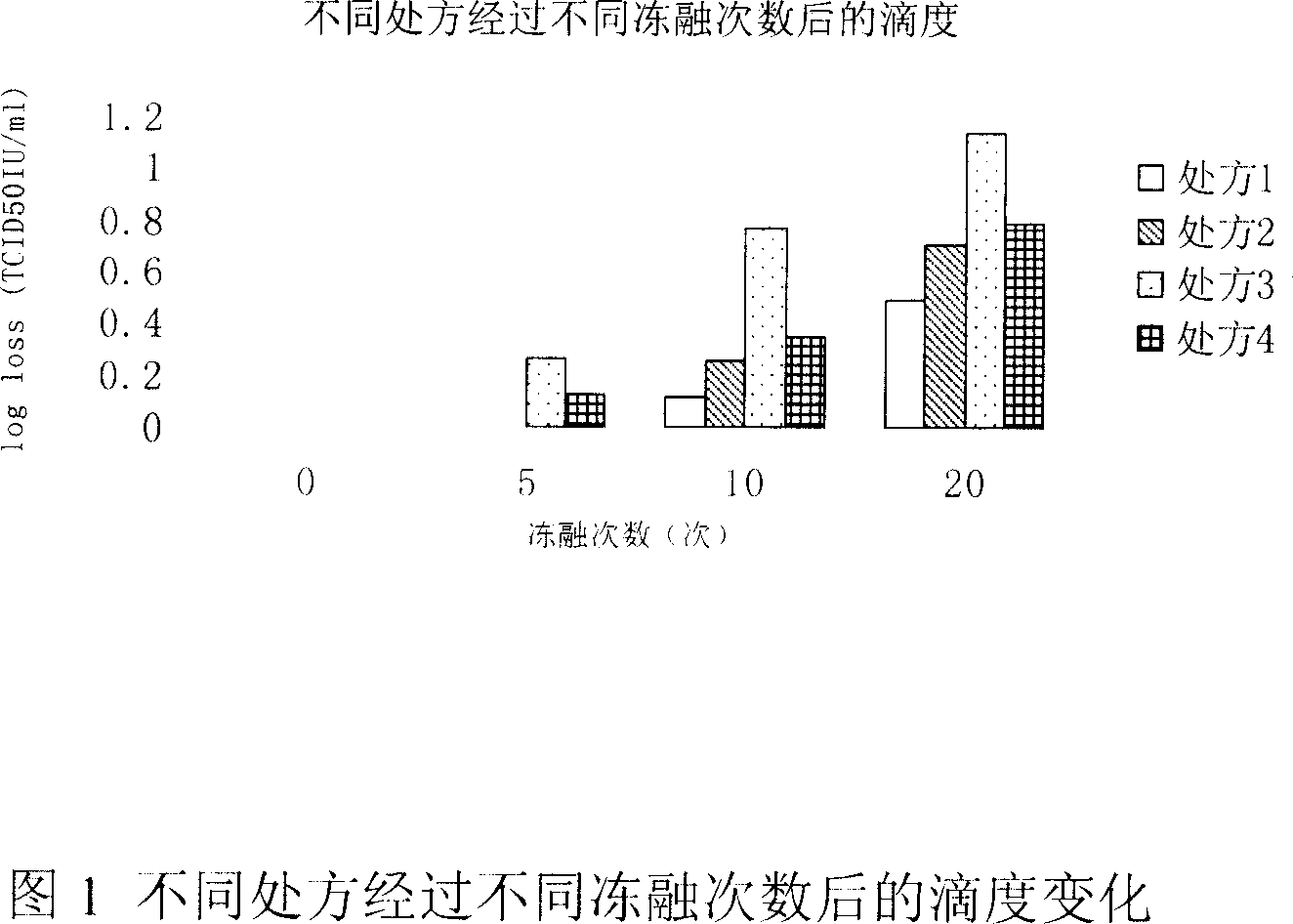
[0030] Embodiment 1: Preparation formula selection experiment
[0031] Recombinant human hepatitis B core antigen adenovirus preparation prescription:
[0032] Prescription 1: KH 2 PO 4 0.41g; K 2 HPO 4 5.59g; NaCl 4.38g;
[0033] MgCl 2 ·6H 2 O 0.20g; Glycerin 100ml; Tween80 0.2ml;
[0034] EDTA 0.03g; ethanol 5ml; pH7.5
[0035] Prescription 2: KH 2 PO 4 0.41g; K 2 HPO 4 5.59g; NaCl 9.00g;
[0036] Histidine 1.55g; Glycerin: 100ml; pH7.5
[0037] Prescription 3: KH 2 PO 4 0.41g; K 2 HPO 4 5.59g; NaCl 4.38gMgCl 2 ·6H 2 O: 0.20g; pH7.5
[0038] Prescription 4: KH2PO4 0.41g; K2HPO4 5.59g; NaCl 9.00g;
[0039] Glycerin 100ml; Tween80: 0.2ml; L-cysteine 1.20g; pH7.5 Preparation:
[0040] Weigh each component according to the formula, add distilled water to about 500ml, stir well, wait until it is completely dissolved, and then make the purified concentration 5×10 11 Add 10ml of vp / ml recombinant human hepatitis B core antigen adenovirus preparation ...
Embodiment 2
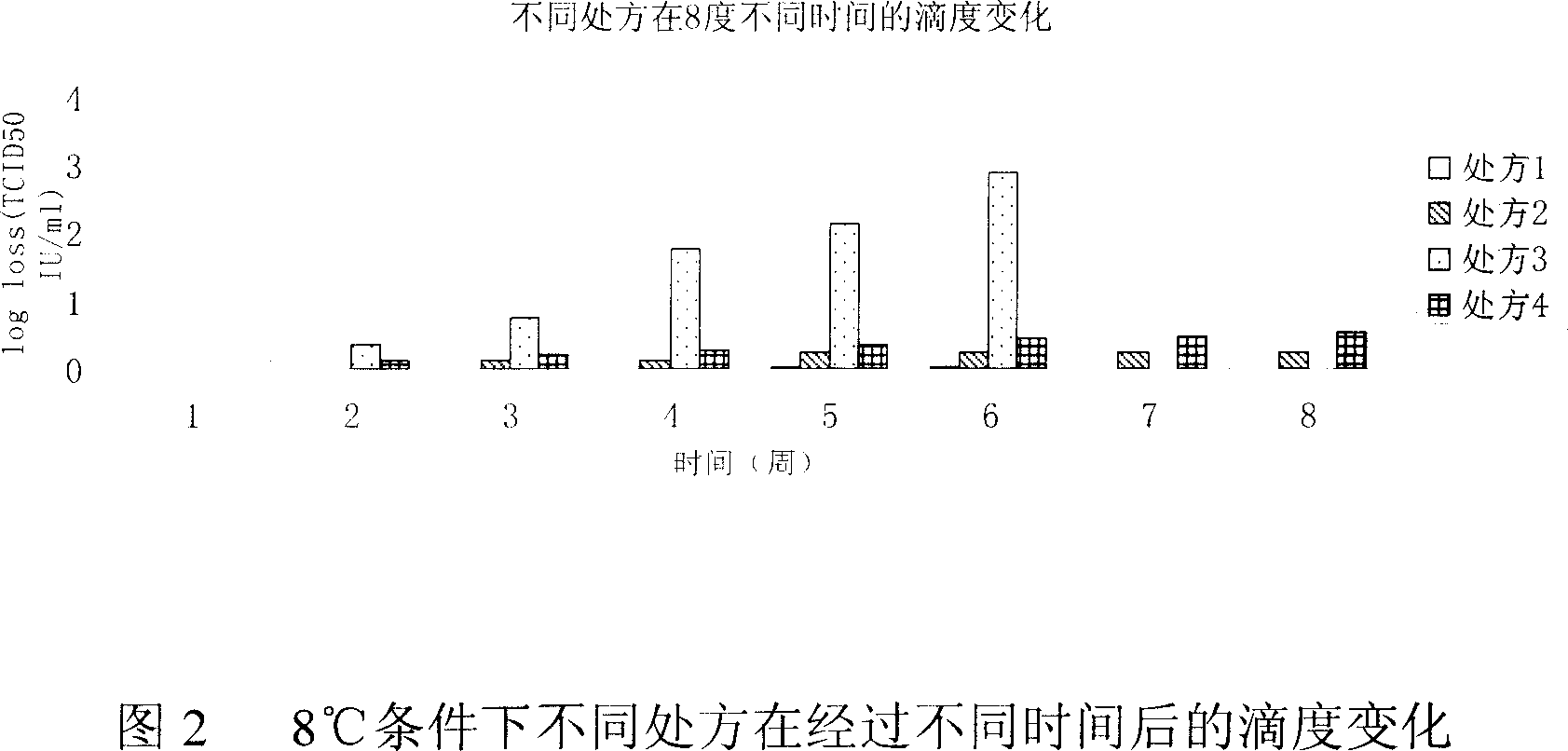
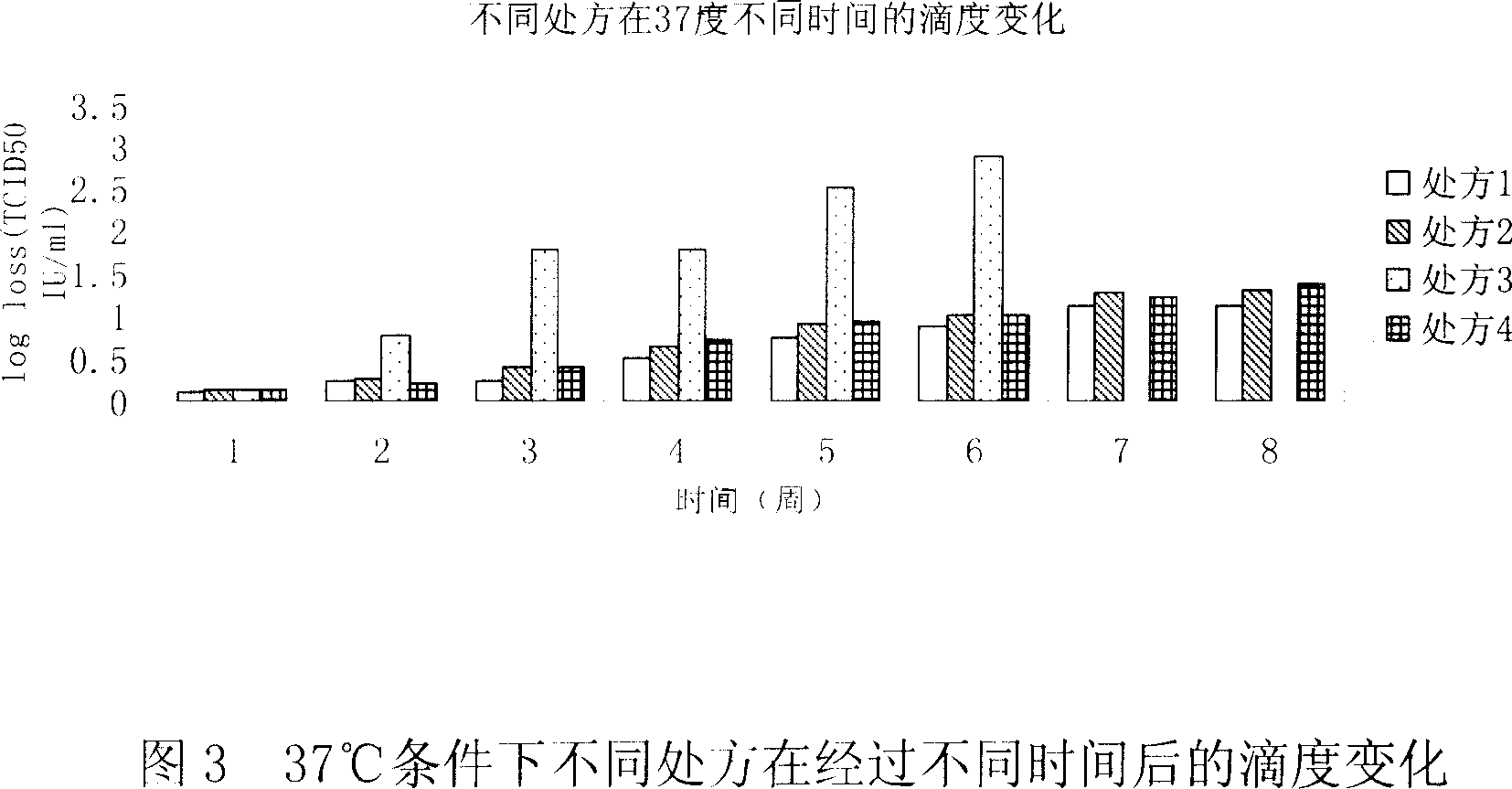
[0044] Embodiment 2: formulation accelerated test (temperature-time test)
[0045] By accelerating the chemical or physical changes of the drug, the stability of the drug is predicted.
[0046] A certain amount of prescription 1 preparations were taken from the same batch of preparations and stored under different temperature conditions: -80°C, -20°C, 8°C, 37°C. The preparations are marked with the batch number and storage temperature. On the day of storage at different temperatures (week 0), samples were taken for testing, and then samples were taken for TCID50 testing according to the time points in the following table.
[0047] Table 1: Detection frequency of different temperature conditions in the accelerated test of preparations
[0048] temperature
[0049] The test results are shown in Figure 4. It can be seen from the figure that the quality of the preparation is stable at -80°C, -20°C and 8°C for 24 weeks, and the test results fluctuate within the measur...
Embodiment 3
[0050] Embodiment 3: long-term stability test
[0051] The long-term test is carried out under the conditions close to the actual storage of the drug, and its purpose is to provide a basis for formulating the effective period of the drug.
[0052] Based on the results of Example 3, this experiment was designed: 8°C was selected for long-term stability experiment. Store three batches of prescription 1 preparations at 8°C, take samples for testing on the day of storage (0 month), and then take samples for TCID50 testing at 1, 3, 6, 9, 12, 18, 24, and 36 months of storage . The test results are shown in Table 2:
[0053] Table 2: Long-term stability test results stored at 8°C: (unit: IU / ml)
[0054] time
[0055] According to the experimental results, see Figure 5. The results show that: the adenovirus liquid preparation made by prescription 1 of the present invention has good stability, and it is stored for 36 months (i.e. 3 years) at 8°C, and the infectivity tite...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com