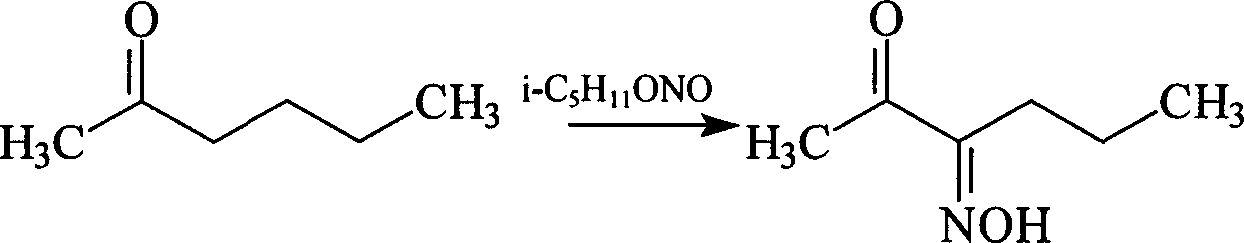Method for synthesis of 2,3-hexanedione monoxime
A technique for the synthesis of hexanedione monoxime, which is applied in oxime preparation, organic chemistry, etc., can solve the problems of low monoxime product yield, and achieve the effects of high reaction yield, easy operation, and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0007] The implementation of the present invention will be further described below.
[0008] Into a 500ml round-bottomed three-necked flask equipped with a stirrer, dropping funnel and thermometer, add 130g (1.3mol) of 2-hexanone and 12ml of dilute hydrochloric acid. Under cooling, start to add 200g (1.7mol) of nitrous acid. Isoamyl ester, after dripping, continue to stir for 2 hours, the temperature is always controlled between 40-50 ℃. The reaction yield was 70%. 2,3-Hexanedione monooxime is an important intermediate for the synthesis of 2,3-hexanedione.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com