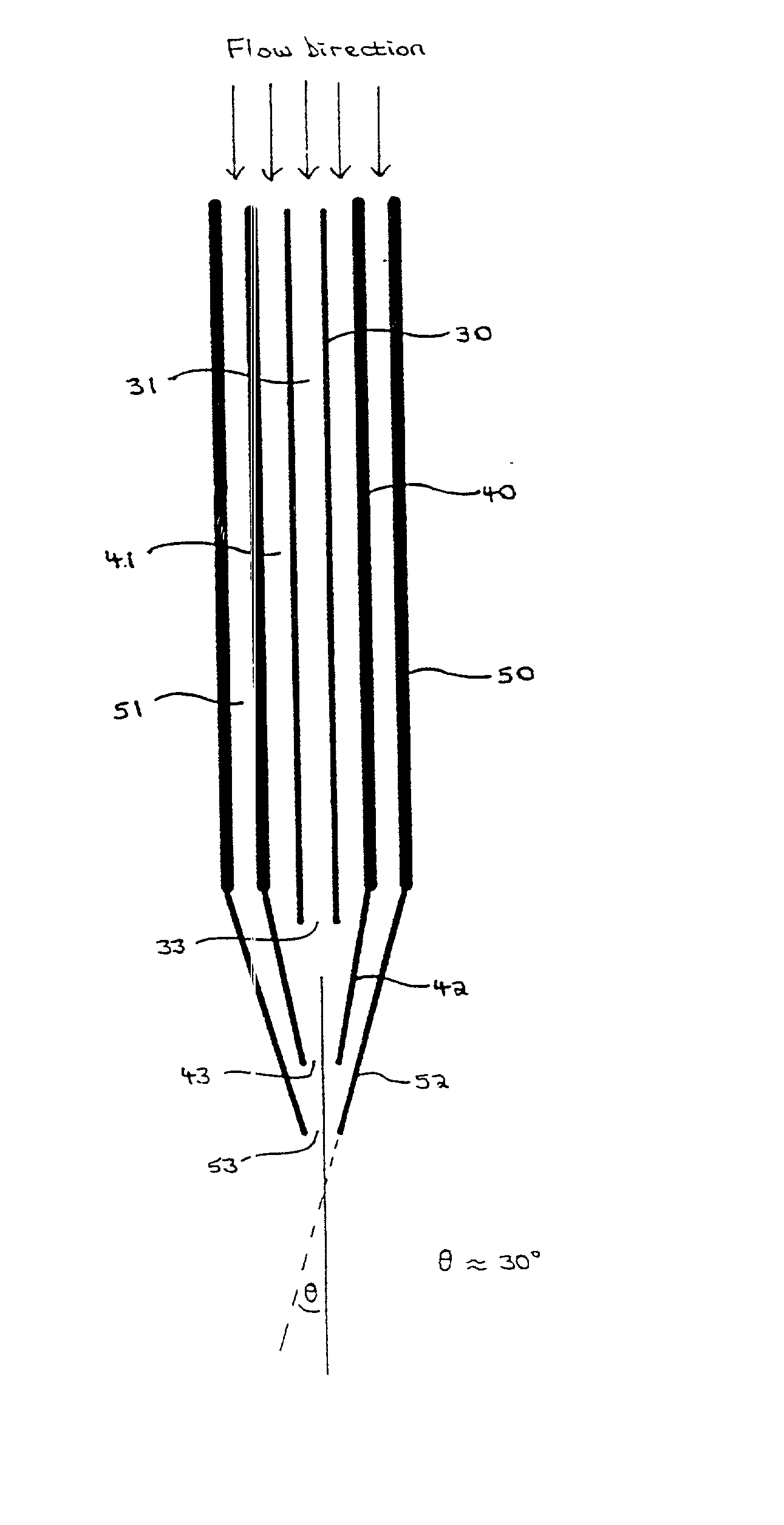
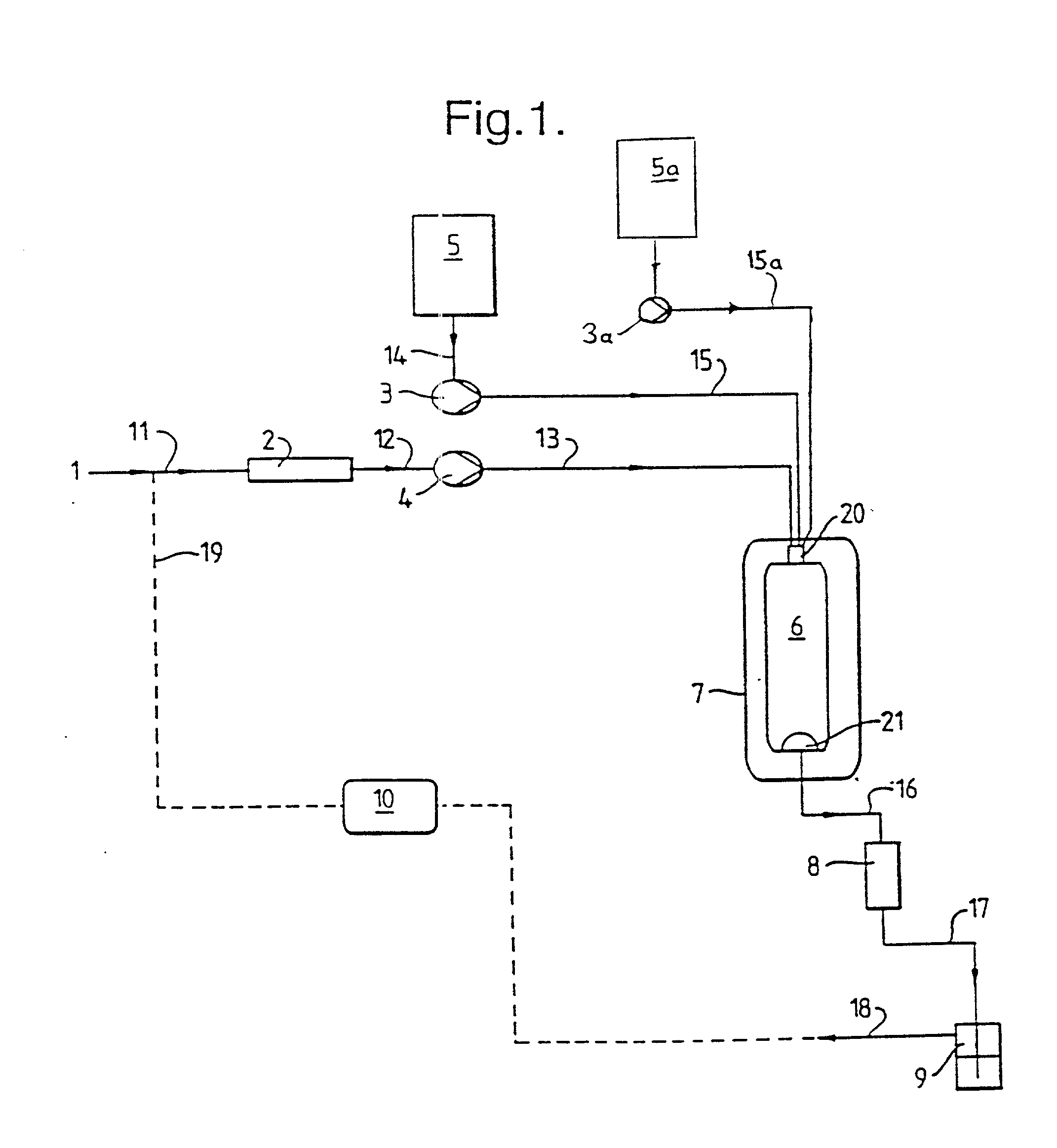
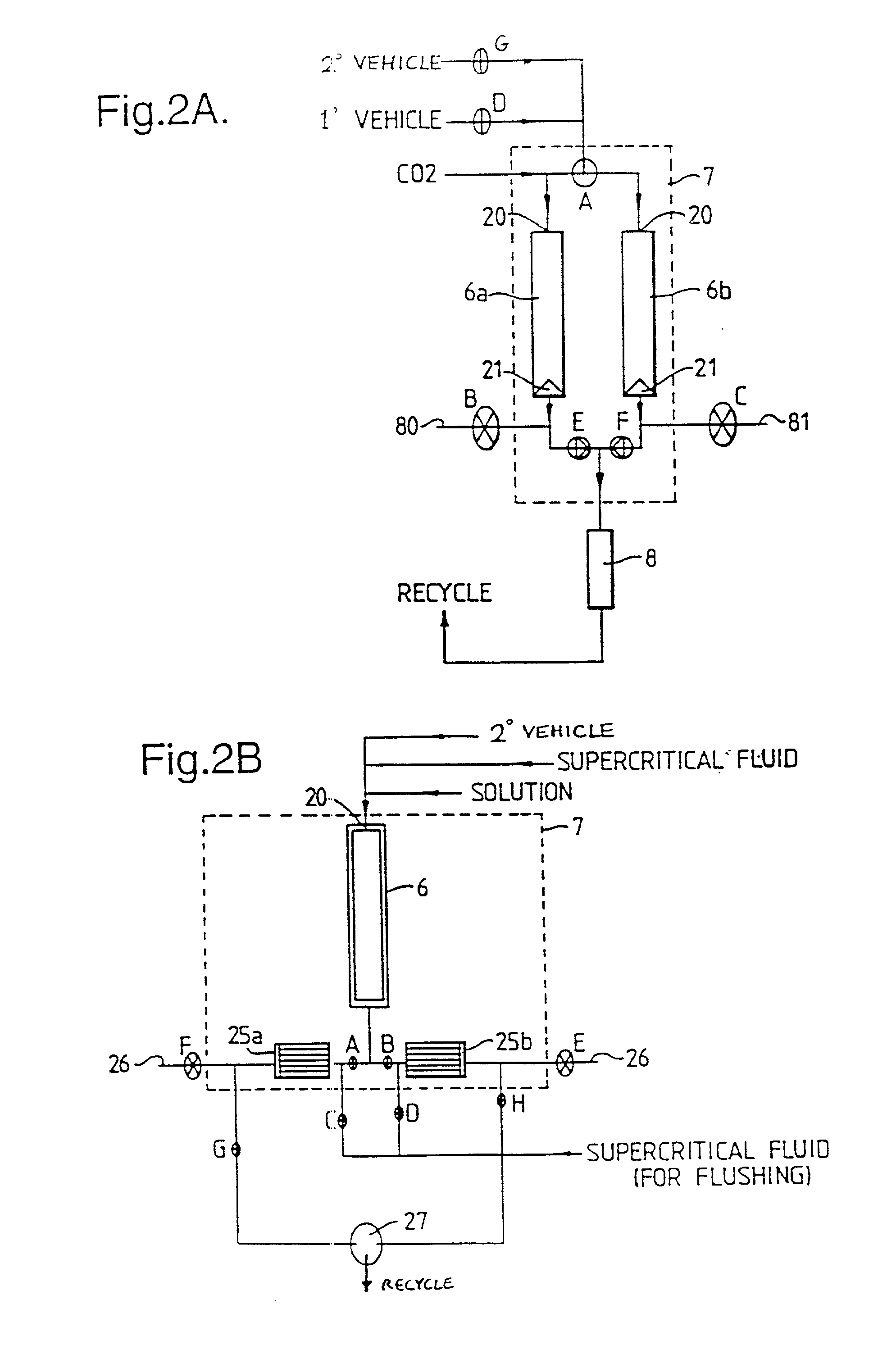
Method and apparatus for the formation of particles
a particle and particle technology, applied in the field of particle particle formation methods and apparatuses, can solve problems such as suffering detrimental effects, and achieve the effect of low solubility of lactos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1 & 2
[0141] Formation of Lactose Particles
[0142] The following examples illustrate the successful and controlled formation of crystalline lactose, using carbon dioxide as a supercritical fluid, despite the very low solubility of lactose in conventional CO.sub.2-soluble organic solvents. According to the present invention, two vehicles were used for the lactose, water as the first and an organic solvent (methanol), which is miscible with water and soluble in supercritical carbon dioxide, as the second.
example 1
[0143] Preparation of Lactose (I)
[0144] In accordance with the invention, a solution of lactose in a relatively small amount of water and a relatively large amount of a second vehicle, methanol, was used. The solution was co-introduced, with supercritical CO.sub.2, into a particle formation vessel of the type shown in FIGS. 1 and 2, through a three-passage nozzle of the type shown in FIG. 3. The pressure and temperature inside the vessel were carefully maintained at the desired operating levels throughout particle formation. It is thought that the miscible water and methanol were extracted together into the supercritical CO.sub.2, despite the insolubility of water in the supercritical fluid.
[0145] 0.3 g of alpha-lactose monohydrate was dissolved in 2 ml de-ionised water, 98 ml of methanol was added to the aqueous solution and the mixture was introduced into the 32 ml particle formation vessel through the three-passage nozzle. The operating conditions were 270 bar and 70.degree. C. i...
example 2
[0146] Preparation of Lactose (II)
[0147] In an experiment similar to that of Example 1, a 0.5% w / v solution of alpha-lactose monohydrate in methanol:water (95:5 v / v) was prepared and delivered to a 50 ml high pressure particle formation vessel via a two-passage nozzle. The working conditions were 150 bar and 50.degree. C. inside the vessel, with a flow rate of 0.7 ml / min for the solution and 9 ml / min for the supercritical CO.sub.2. The collected product was a free flowing, fine white powder. FIGS. 7 and 8 show an SEM micrograph and XRD pattern respectively for this product.
[0148] The SEM micrographs for the products of Examples 1 and 2 reveal a marked difference in the shape of the lactose particles prepared under the different operating conditions. The XRD patterns indicate the crystalline nature of the products.
[0149] As can be seen from these examples, the present invention provides an extremely effective technique for the controlled formation of lactose particles using supercrit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com