Diacylglycerol o-acyltransferase 2alpha (DGAT2alpha)
a technology of acylglycerol and acyltransferase, applied in the field of acyltransferases, can solve the problem of reducing the period of time that the therapy is effectiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0129] I. Existence of DGAT2.alpha.
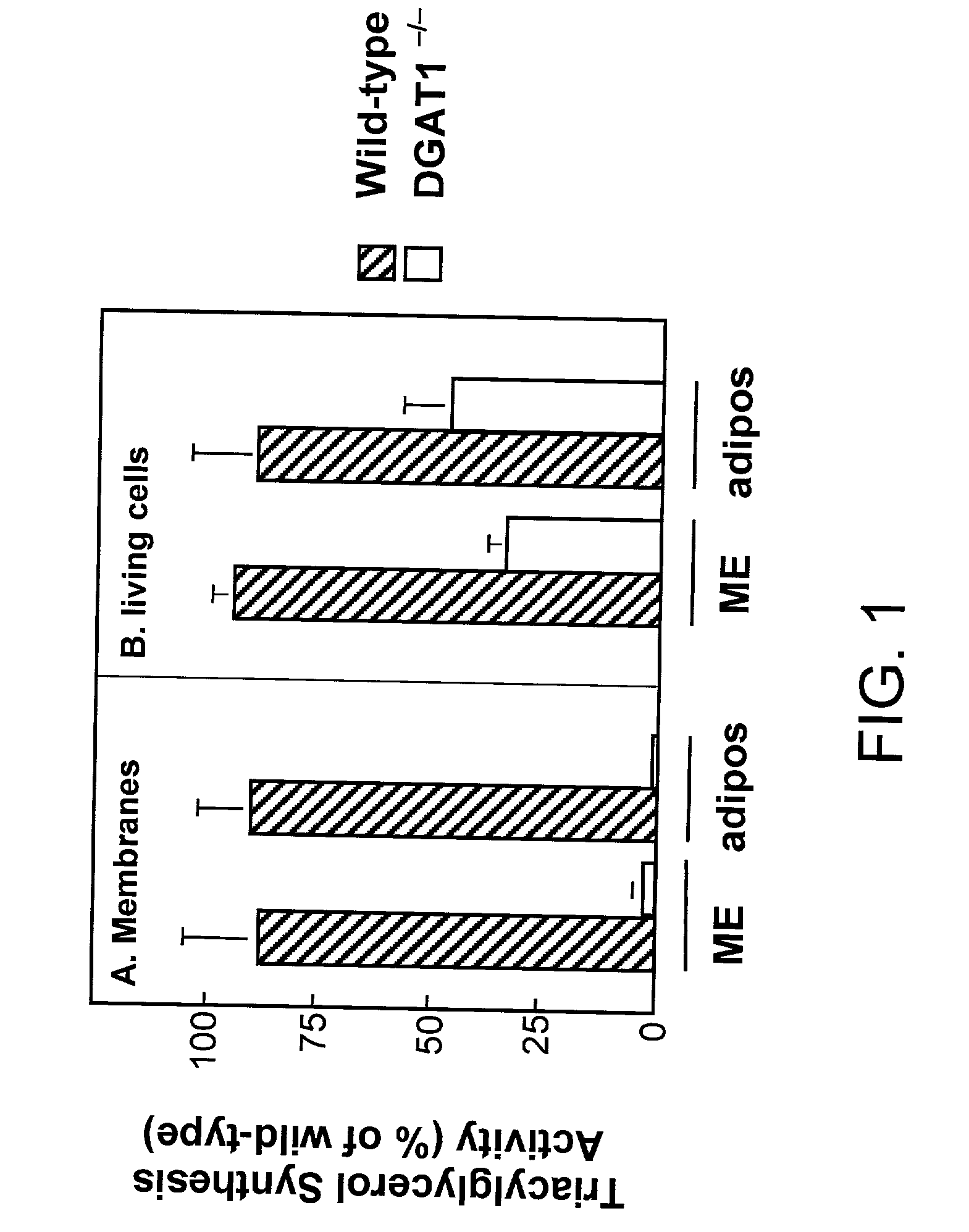
[0130] A. Mice (DGAT1- / -) lacking DGAT, as described in WO 99 / 67268 are lean and resistant to diet-induced obesity, but are still capable of synthesizing triglycerides (TG) and have normal plasma TG levels. However, DGAT activity is virtually absent in membrane preparations from DGAT1- / - tissues (Smith et al., Nat. Genet. 2000 (25), 87-90). Using pulse assays in living cells, we measured that the residual TG synthesis activity in DGAT 1- / -Mouse Embryonic Fibroblasts (MEF) or adipocytes was about 40% of that in wild-type cells. The results are graphically depicted in FIGS. 1A and 1B. In FIG. 1A the membrane fraction isolated from MEF or adipocytes of wild-type or DGAT1- / - mice was used as the enzyme source in DGAT assays in vitro. In FIH. 1B living cells were pulse-labeled with [.sup.14C]oleic acid for 24 hours and [.sup.14C] incorporation in the TG fraction was measured.
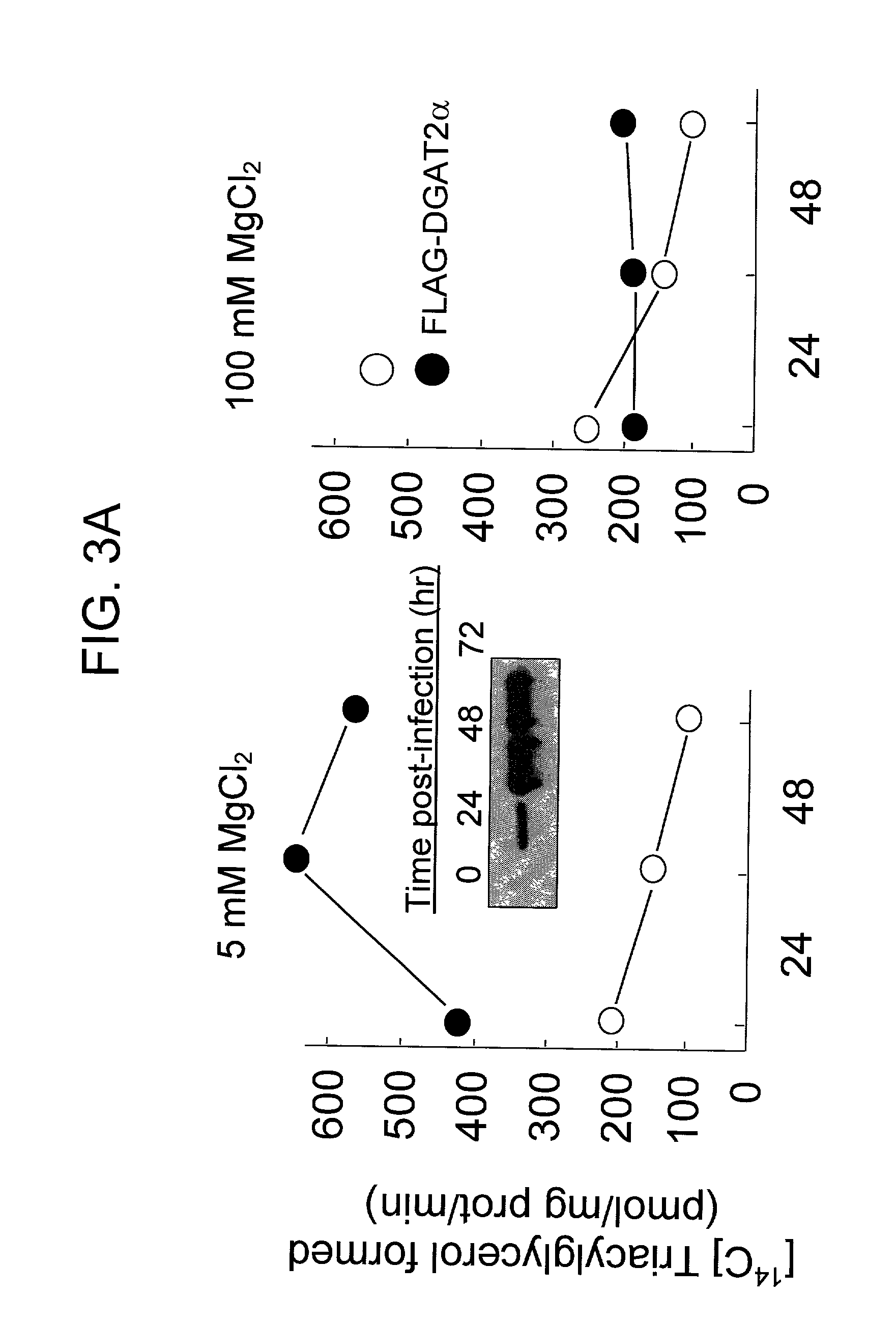
[0131] In further assays, increased DGAT activity was observed in DGAT.sup.-1-m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| compositions | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com