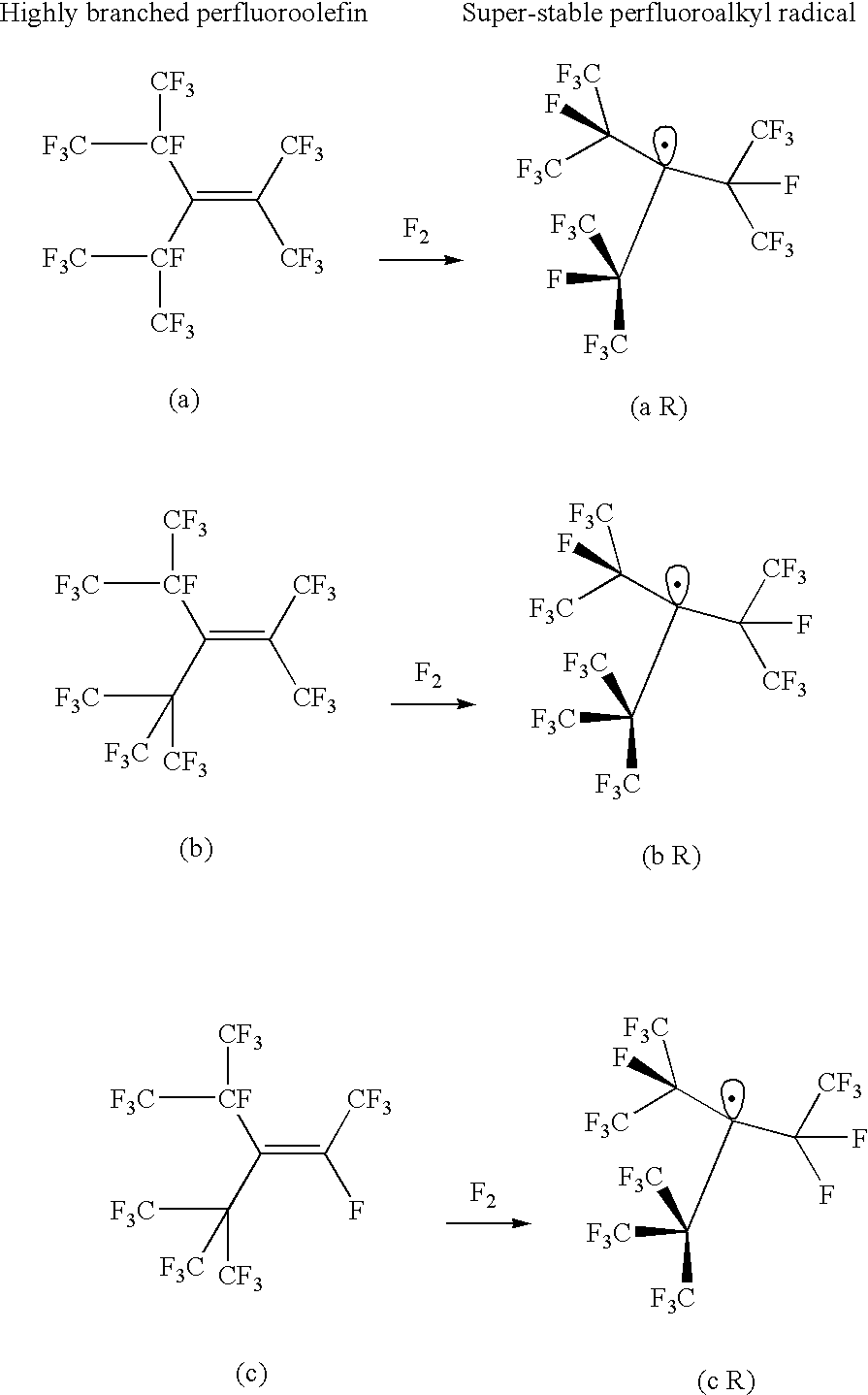
Highly branched perfluoroolefins, super-stable perfluoroalkyl radicals and production methods thereof
a perfluoroalkyl radical, superstable technology, applied in the field of high-branched perfluoroolefins, superstable perfluoroalkyl radicals and production methods thereof, can solve the problems of difficult to synthesize sterically complex perfluoroolefins, no other methods for perfluorosynthesis, and inapplicability of synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Perfluoro(2,4-dimethyl-3-isopropyl-2-pentene)
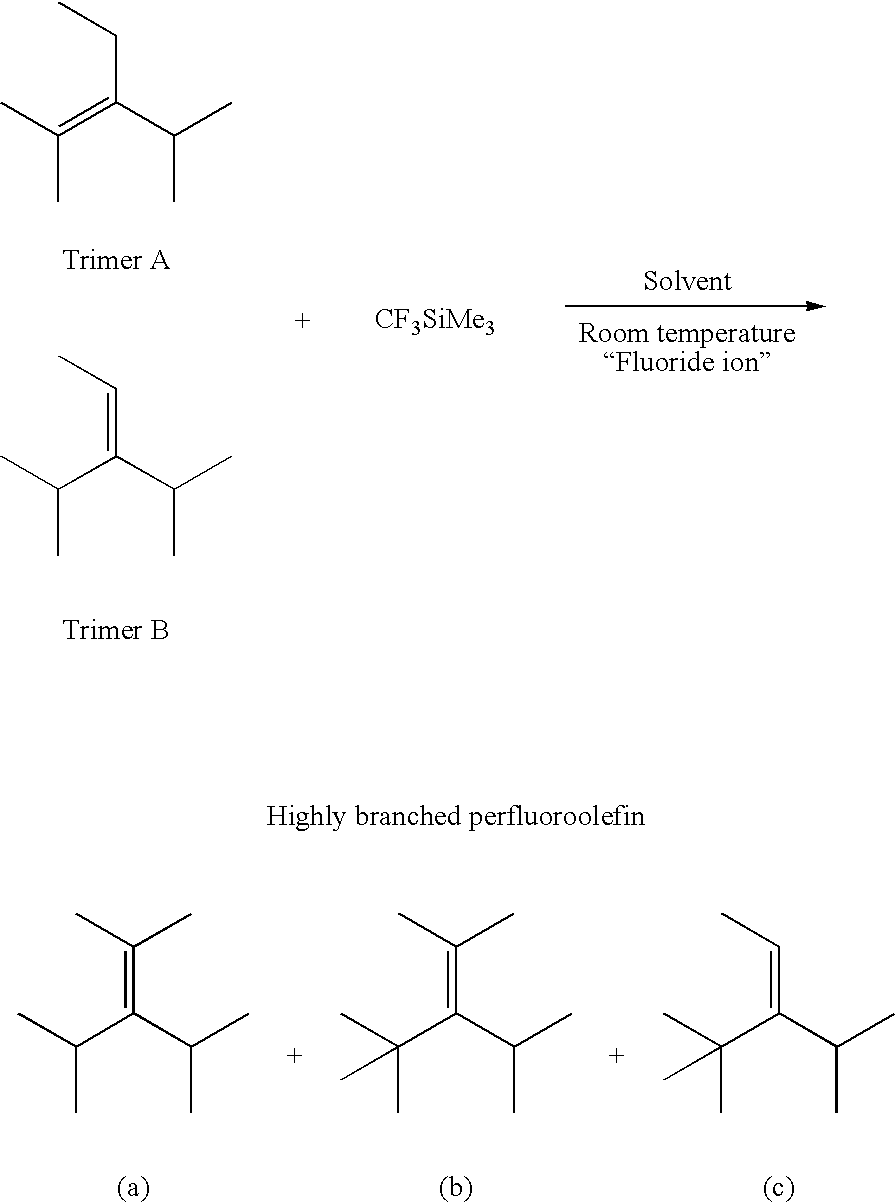
[0103] 1 mmol (450 mg) of a hexafluoropropene trimer mixture (containing 10% by weight of perfluoro(3-ethyl-2,4-dimethyl-2-pentene)) whose main component was perfluoro(4-methyl-3-isopropyl-2-pentene) and 1.1 mmol (23.4 mg) of trifluoromethyltrimethylsilane were weighed into a 10-ml fluororesin-made reaction container, and 1 ml of dimethyl formamide and 0.3 mmol of acidic potassium fluoride (KHF.sub.2) were added. A fluororesin-made magnetic stirrer was placed therein, and the mixture was stirred vigorously for 1 hour at room temperature. The transparent lower perfluorocarbon layer was separated into each component by preparative gas chromatography (using a column whose mobile phase was Fomblin), and the structure was identified by .sup.19F-NMR. The yield of a main product perfluoro(2,4-dimethyl-3-isopropyl-2-pentene) calculated on the basis of the ratio of the peak areas in the gas chromatography using a capillary column (NB-...
example 2
Synthesis of Perfluoro(2,4-dimethyl-3-isopropyl-2-pentene)
[0111] 1 mmol (450 mg) of a hexafluoropropene trimer mixture (containing 10% by weight of perfluoro(3-ethyl-2,4-dimethyl-2-pentene)) whose main component was perfluoro(4-methyl-3-isopropyl-2-pentene) and 4.0 mmol (568.8 mg) of trifluoromethyltrimethylsilane were weighed into a 10-ml fluororesin-made reaction container, and 1 ml of dimethyl formamide and 0.1 mmol of acidic potassium fluoride (KHF.sub.2, 7.8 mg) were added. A fluororesin-made magnetic stirrer was placed therein, and the mixture was stirred vigorously for 1 hour at room temperature. The transparent lower perfluorocarbon layer was analyzed by gas chromatography using a capillary column (NB-1, 0.25 .mu.m, 1.5 mm ID.times.60 m). The yields of the main product perfluoro(2,4-dimethyl-3-isopropyl-2-pentene) and perfluoro(2,4,4-trimethyl-3-isopropyl-2-pentene) were 63.5% by weight and 36.5% by weight, respectively.
[0112] Accordingly, it was revealed that the reaction c...
example 3
Selective Synthesis of Perfluoro(2,4-dimethyl-3-isopropyl-2-pentene)
[0113] 1 mmol (450 mg) of a hexafluoropropene trimer mixture (containing 10% by weight of perfluoro(3-ethyl-2,4-dimethyl-2-pentene)) whose main component was perfluoro(4-methyl-3-isopropyl-2-pentene) and 2.0 mmol (284.4 mg) of trifluoromethyltrimethylsilane were weighed into a 10-ml fluororesin-made reaction container, then 1 ml of 1,3-dimethyl-2-imidazol-idinone and 0.1 mmol of acidic potassium fluoride (KHF.sub.2, 7.8 mg) were added therein. A fluororesin-made magnetic stirrer was placed and the mixture was stirred vigorously for 1 hour at room temperature, and then 1.0 mmol of trifluoromethyltrimethylsilane (142.2 mg) and 0.1 mmol of acidic potassium fluoride (KHF.sub.2, 7.8 mg) were added and the mixture was further stirred vigorously for 1 hour at room temperature. After completion of the reaction, the transparent lower perfluorocarbon layer was analyzed by gas chromatography using a capillary column (NB-1, 0.2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| room temperature | aaaaa | aaaaa |
| reaction temperature | aaaaa | aaaaa |
| reaction temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com