New hydrofluoro ether and its preparation method
A new technology of hydrofluoroether, which is applied in the field of new hydrofluoroether and its preparation, to achieve the effect of easy separation and purification, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example I
[0033] Example I: Effect of Solvent
example I-1
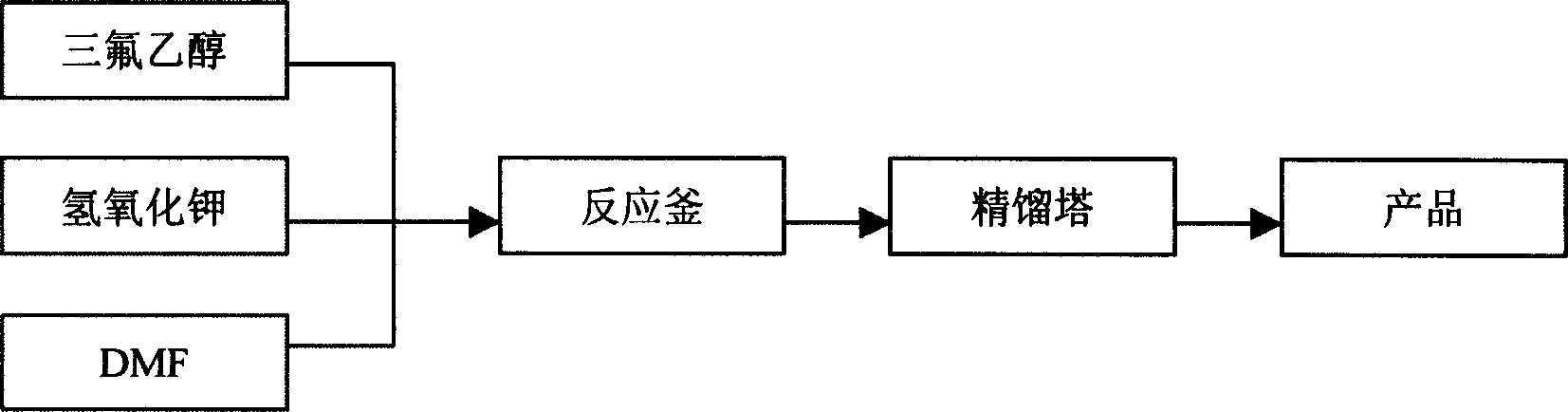
[0035] Dissolve 11.2g (0.2mol) of potassium hydroxide in 50g (0.5mol) of trifluoroethanol, add 100 grams of DMF solvent, seal in a 500mL autoclave, cool at low temperature, and introduce 130 grams (1.58mol) of trifluoroethylene in vacuum , heated to 78°C, and reacted for 6.5 hours. 89.2 grams of crude product were obtained by distillation, with a yield of 94.8%.
example I-2
[0037] Dissolve 11.2g (0.2mol) of potassium hydroxide in 50g (0.5mol) of trifluoroethanol, add 100 grams of DMSO solvent, seal in a 500mL autoclave, cool at low temperature, and introduce 130 grams (1.58mol) of trifluoroethylene in vacuum , heated to 78°C, and reacted for 6.5 hours. 87.3 grams of crude product were obtained by distillation, with a yield of 92.8%.
[0038] In the comparison of example I-1 and I-2, the yield is slightly higher than that of DMSO with DMF as the solvent. At the same time, during the distillation and purification process, DMSO has a partial decomposition phenomenon, which increases the loss of solvent and affects the purity of the product.
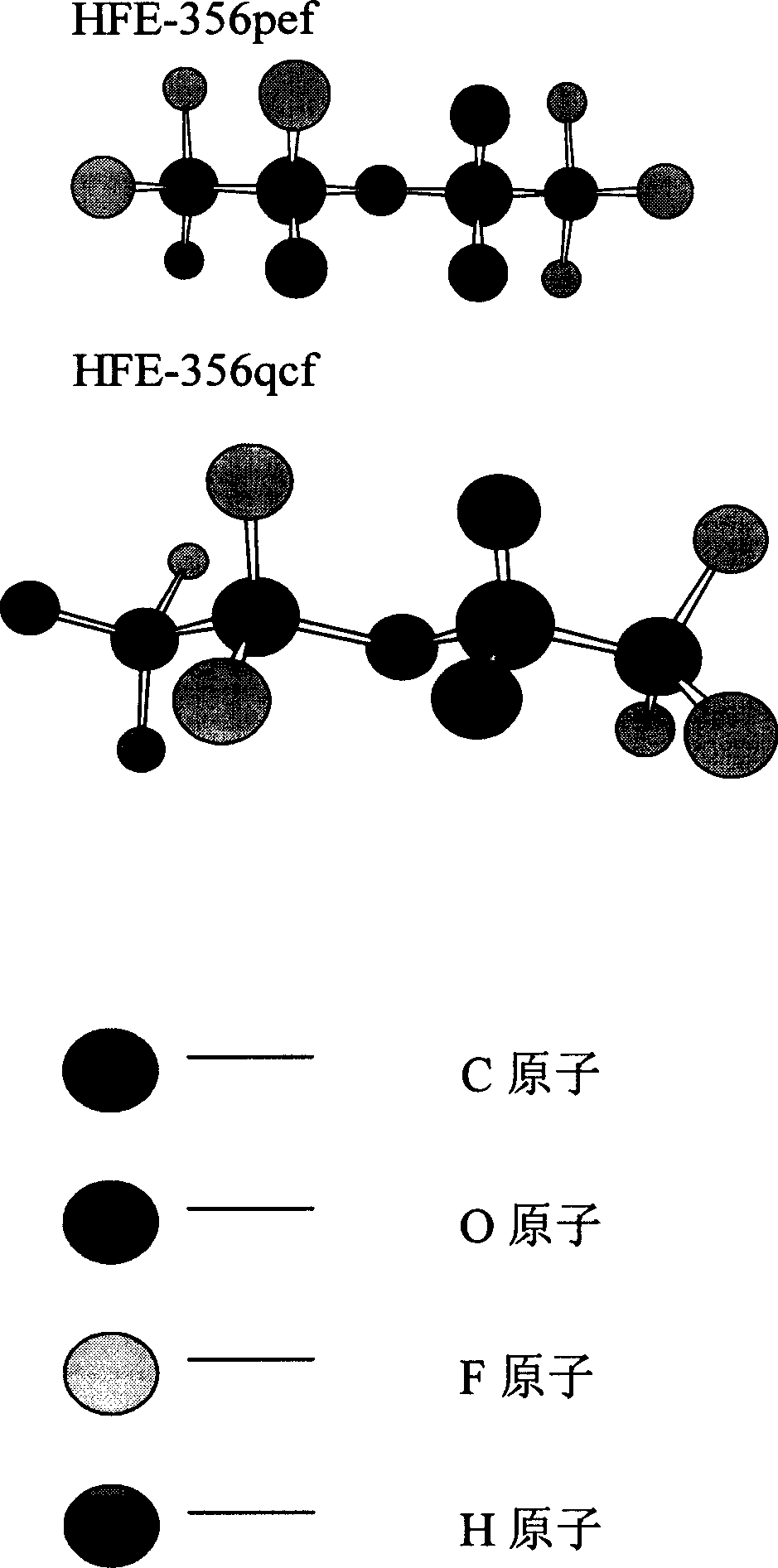
[0039] Because trifluoroethylene is a geometrically asymmetric structure, two isomers are formed when it is added to alcohol:
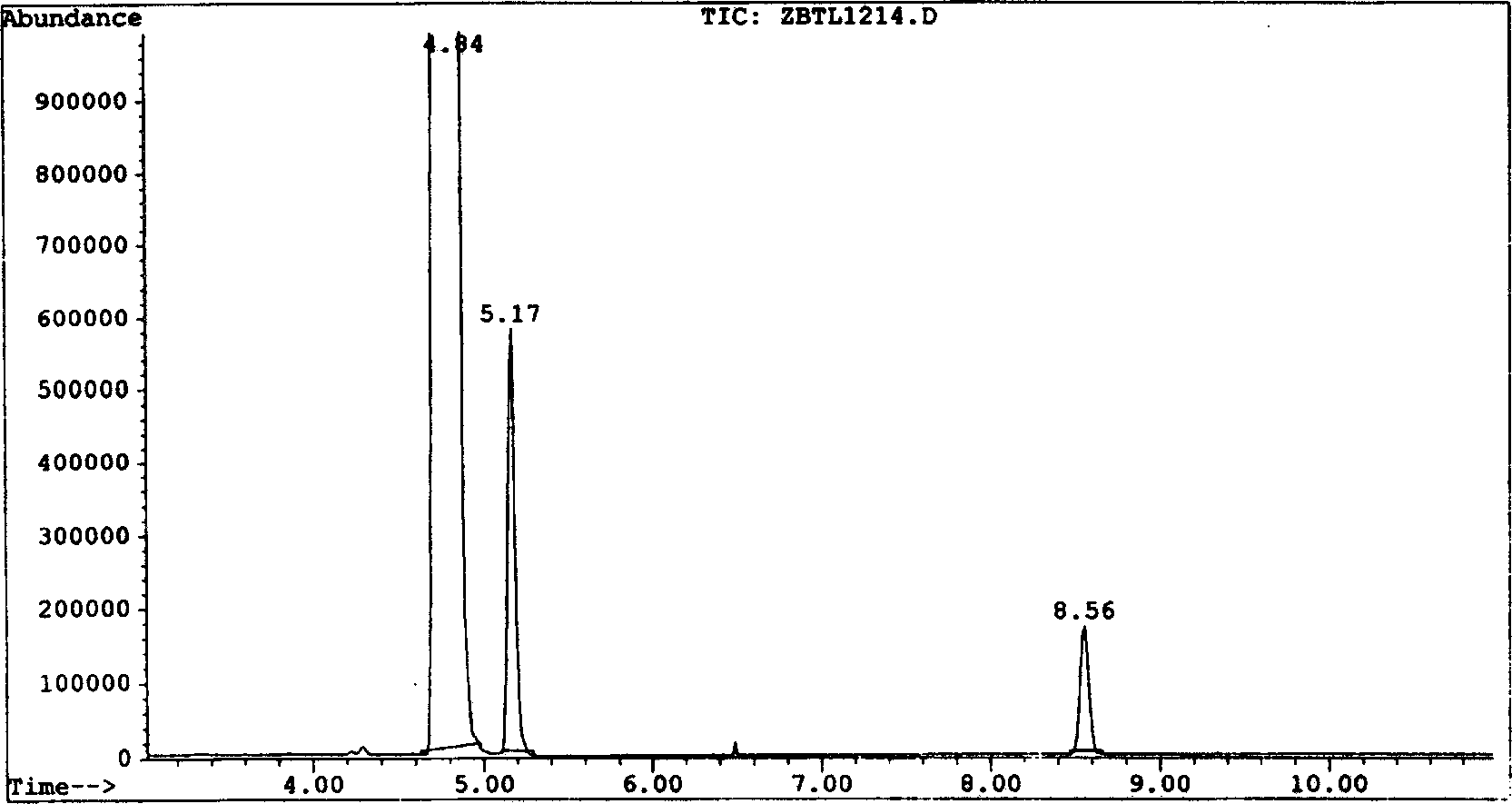
[0040] Chromatographic analysis shows that: when DMF and DMSO are used as solvents for the reaction, the product obtained by the addition of trifluoroethylene and trifluoroetha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com