Electrolytic cell and electrodes for use in electrochemical processes
a technology of electrochemical processes and electrolysis cells, applied in the field of electrochemical cells, can solve the problems of significant energy cost reduction, hydrochloric acid production problem, and inability to be dumped into sewers and wastewater outlets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
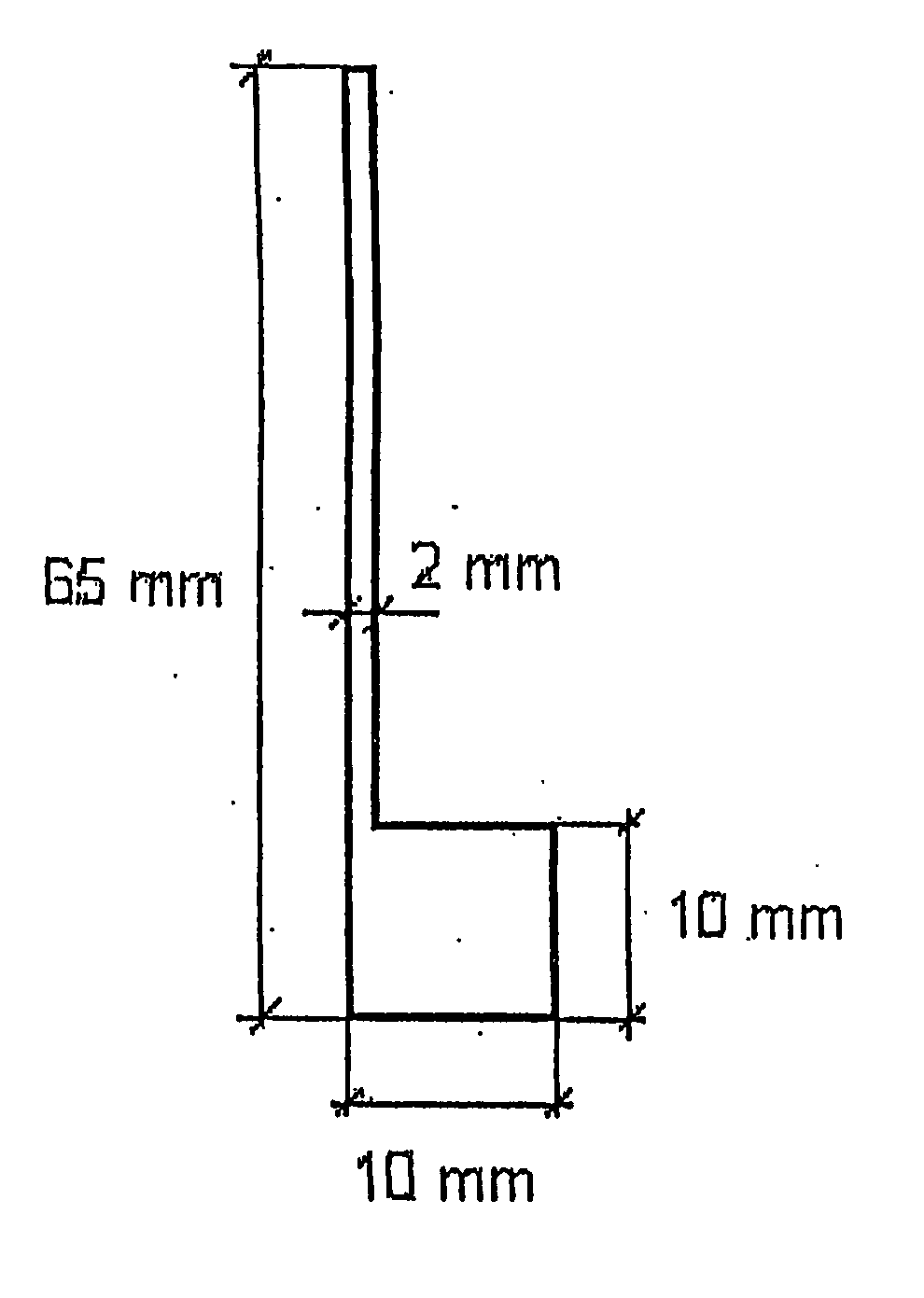
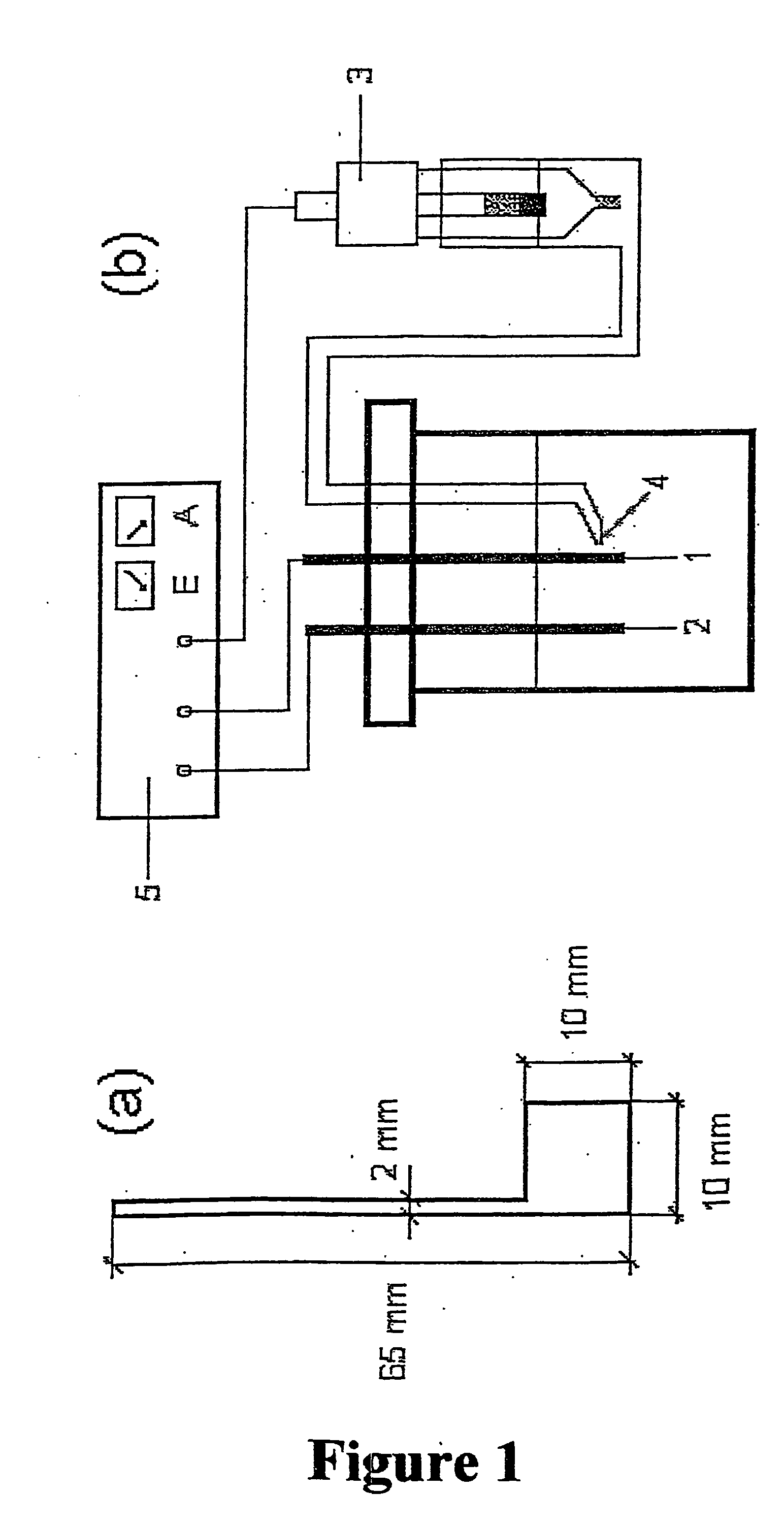
[0030] The present invention relates to an electrolytic cell with electrodes useful in electrochemical processes. The present invention also relates to cathodes and anodes useful for hydrogen evolution in cells for the electrolysis of hydrochloric acid solutions, alkali metal halide solutions, and alkaline solutions.
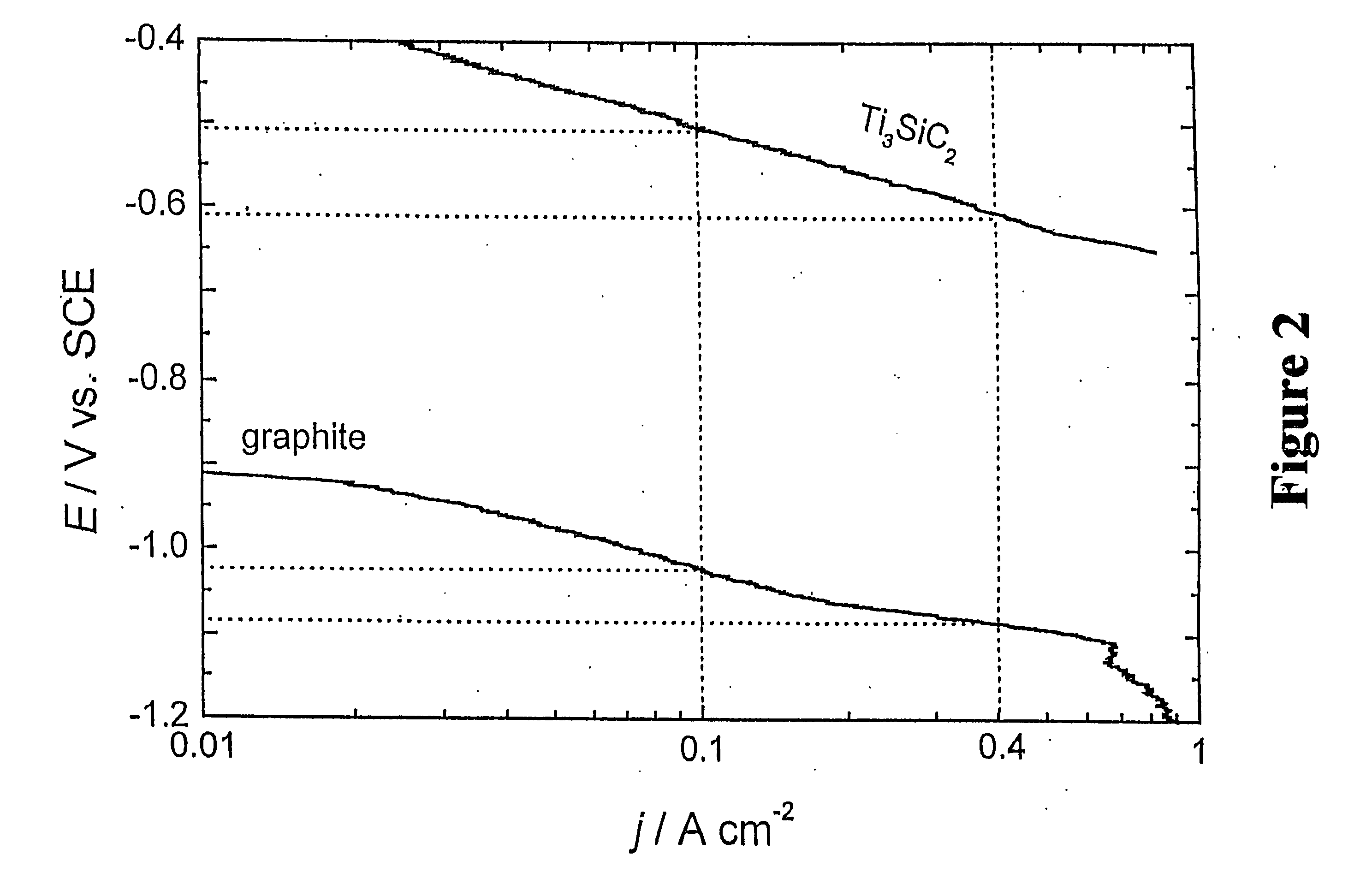
[0031] The cathodes of the present invention comprise either a bulk or plasma sprayed electrocatalytic ceramic or intermetallic material of the composition Mn+1AXn (n=1, 2, 3) wherein M is a metal selected from group IIIB, IVB, VB, VIB or VIII of the periodic table of elements and / or a mixture thereof; wherein A is selected from group IIIA, IVA, VA or VIA of the periodic table of elements and / or a mixture thereof; and wherein X is carbon and / or nitrogen. In a preferred embodiment the electrode comprises Ti3SiC2. Any of these materials or their solid solutions may be activated by a thermally prepared coating of a solid solution of TiO2 and RuO2 or TiO2, RuO2 and IrO2, wh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| grain size | aaaaa | aaaaa |
| corrosion rate | aaaaa | aaaaa |
| thicknesses | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com