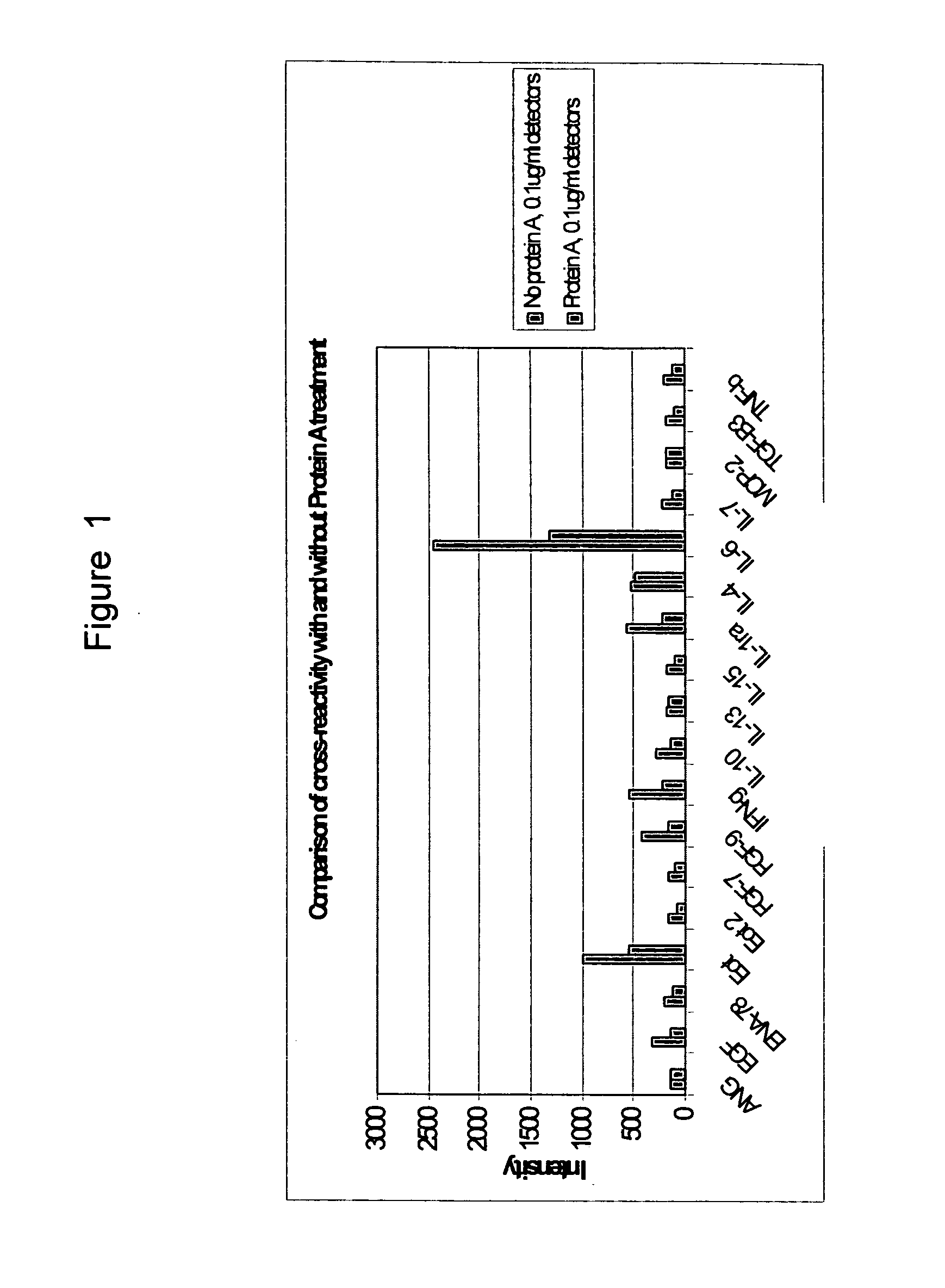
Suppression of cross-reactivity and non-specific binding by antibodies using protein A
a technology of protein a and anti-cross-reactivity, applied in the field of antibody-antibody interaction, can solve the problems of increasing the likelihood of non-specific signals, more troublesome unintended non-specific reactions, and expensive and time-consuming preparation of purified fab fragments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The present invention relates to a blocked immunoglobulin comprising an antibody portion and a Protein A portion. In specific embodiments, the antibody portion comprises at least one light chain variable region of an antibody and / or at least one heavy chain variable region of an antibody. Commonly, there will be at least two light chain and two heavy chain regions, which may include both constant and variable regions.
[0028] With the advent of methods of molecular biology and recombinant technology, it is now possible to produce antibody molecules by recombinant means and thereby generate gene sequences that code for specific amino acid sequences found in the polypeptide structure of the antibodies. Such antibodies can be produced by either cloning the gene sequences encoding the polypeptide chains of said antibodies or by direct synthesis of said polypeptide chains, with in vitro assembly of the synthesized chains to form active tetrameric (H2L2) structures with affinity for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com