Antitumor preparation and preparation method for same
An anti-tumor and preparation technology, applied in the field of anti-tumor preparations and their preparation, can solve problems such as large side effects, and achieve the effects of reducing the dosage of medicine, reducing the cost of medicine and having a wide range of sources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1. Preparation of the anti-tumor preparation of the present invention.
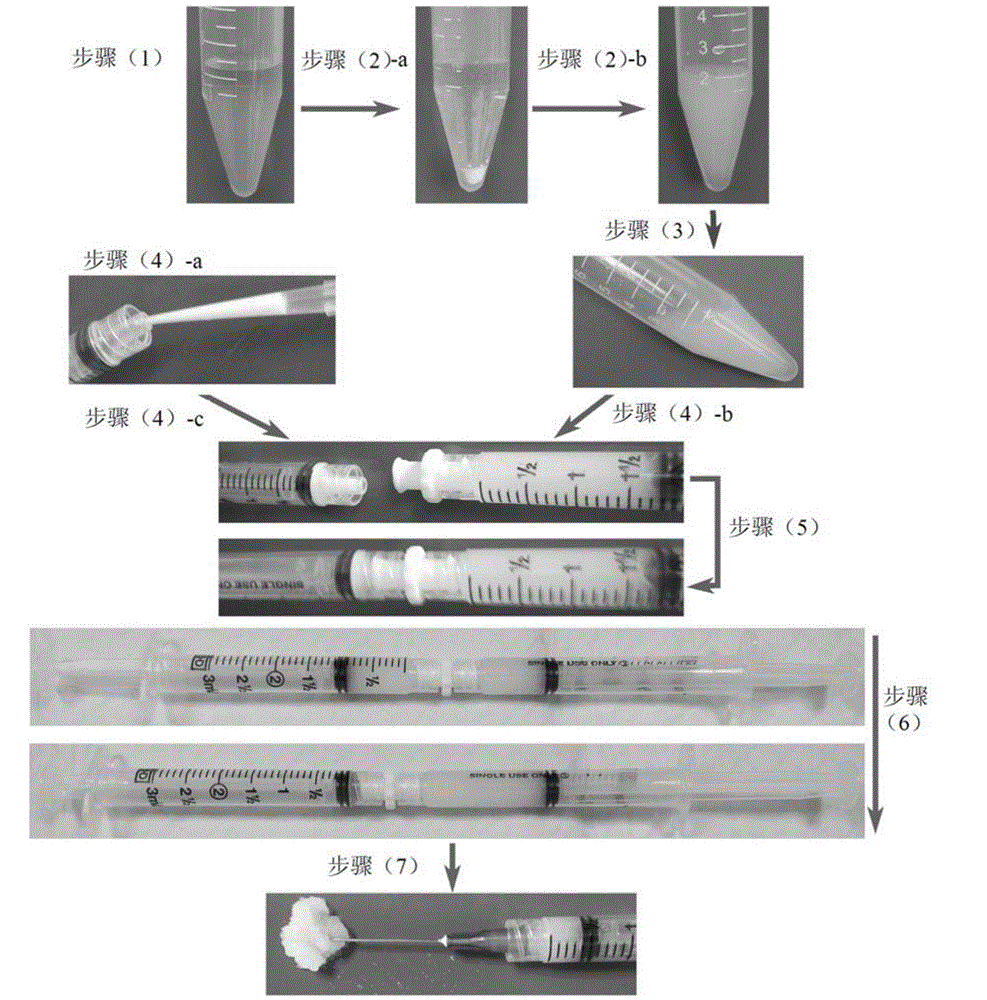
[0039] can follow as figure 1 The scheme shown prepares the antineoplastic formulations of the present invention.
[0040] Step (1), prepare 10-40mg / mL high molecular weight (SLG100, Mw=200,000–300,000g / mol) and low molecular weight (SLG20, Mw=75,000–220,000g / mol) respectively with physiological saline, FMC Biopolymer Company, USA Philadelphia) alginate solution, stirred overnight at 4° C. with a magnetic stirrer, and sterilized by filtration with a filter with a pore size of 0.22 μm. Take high and low molecular weight alginate solutions and mix them in a ratio of 1:3 and mix well;
[0041] Step (2)-a, weighing 3.75-7.5 mg of celecoxib powder (LC Laboratory, LC Laboratories, Massachusetts, USA) per milliliter of alginate solution, and mixing;
[0042] In step (2)-b, use a probe-type ultrasonic instrument (QSONICA, USA) to sonicate for 60 seconds with a power of 60W, so that the celecoxib...
Embodiment 2
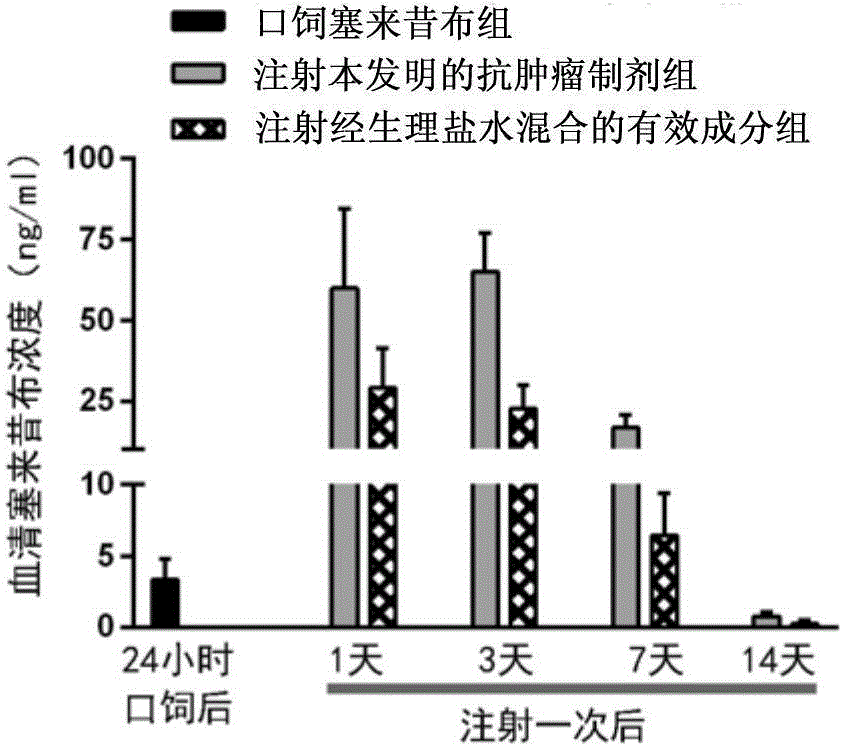
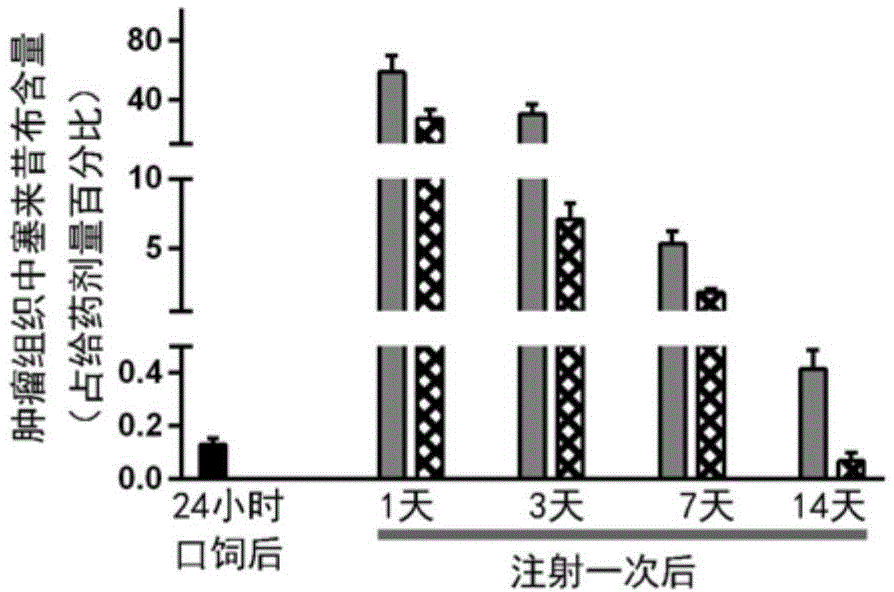
[0050] Example 2: The sustained release effect and drug effect extension effect of the anti-tumor preparation provided by the present invention in vivo
[0051] 1. Prepare the anti-tumor preparation according to the same method as in Example 1, so that each milliliter of the anti-tumor preparation contains 500 ug of PD-1 blocking antibody and 3125 ug of celecoxib.
[0052] C57BL / 6 mice were purchased from Beijing Huafukang Biotechnology Co., Ltd., each weighing about 25 grams. B16-F10 melanoma cells were purchased from the American Type Culture Collection.
[0053] 2. Construction of mouse B16 melanoma model.
[0054] B16-F10 melanoma cells are recognized as the tumor cells with the strongest invasive ability, and each C57BL / 6 mouse was subcutaneously injected with 2.5×10 4 After a single B16-F10 melanoma cell, a well-formed melanoma in situ can be generated after 1 week, and then grow rapidly and cause the death of the mouse. The C57BL / 6 mouse melanoma model is a mature an...
Embodiment 3
[0069] Example 3 The anti-tumor preparation of the present invention can promote the production of active CD8 T cells
[0070] 1. Prepare the anti-tumor preparation according to the same method as in Example 1, so that each milliliter of the anti-tumor preparation contains 500 ug of PD-1 blocking antibody and 3125 ug of celecoxib.
[0071] 2. Construct the same mouse melanoma model as in Example 2.
[0072] One week after C57BL / 6 mice were inoculated with melanoma cells, set up the following 4 groups of mice:
[0073]
[0074] After each group of mice was treated as above, the percentage of active CD8 cells in the tumor microenvironment was detected by flow cytometry one week later.
[0075] Figure 3A with Figure 3B The detection results of CD8 T cells that can effectively kill tumor cells in the tumor microenvironment are shown. It shows that the administration of the anti-tumor preparation group of the present invention (formed by encapsulating PD-1 antibody and celec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com