Method of doping organic semiconductors with quinone derivatives and 1, 3, 2 - dioxaborine derivatives
a technology of organic semiconductors and dioxaborine, which is applied in the direction of non-metal conductors, sustainable manufacturing/processing, final product manufacturing, etc., can solve the problems of high control and regulation expenses, inability to precisely control the manufacturing process in large technical production plants or those on a technical scale, and disadvantages of compounds previously investigated, so as to facilitate the processing of organic semiconductors and reduce volatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0389] Doping of ZnPc with N,N′-dicyano-2,3,5,6-tetrafluoro-1,4-quinonediimine (F4DCNQI)
[0390] The evaporation temperature T(evap.) is 85° C. The two components matrix and dopant were deposited from vapor under vacuum in a ratio of 50:1. Here the conductivity is 2.4×10−2 s / cm. Results are shown in FIG. 1 and Table 1 below.
TABLE 1Layer ThicknessCurrent(nm)(nA)569.0510400.915762.5201147251503.2301874.4352233.4402618453001.5503427
example 2
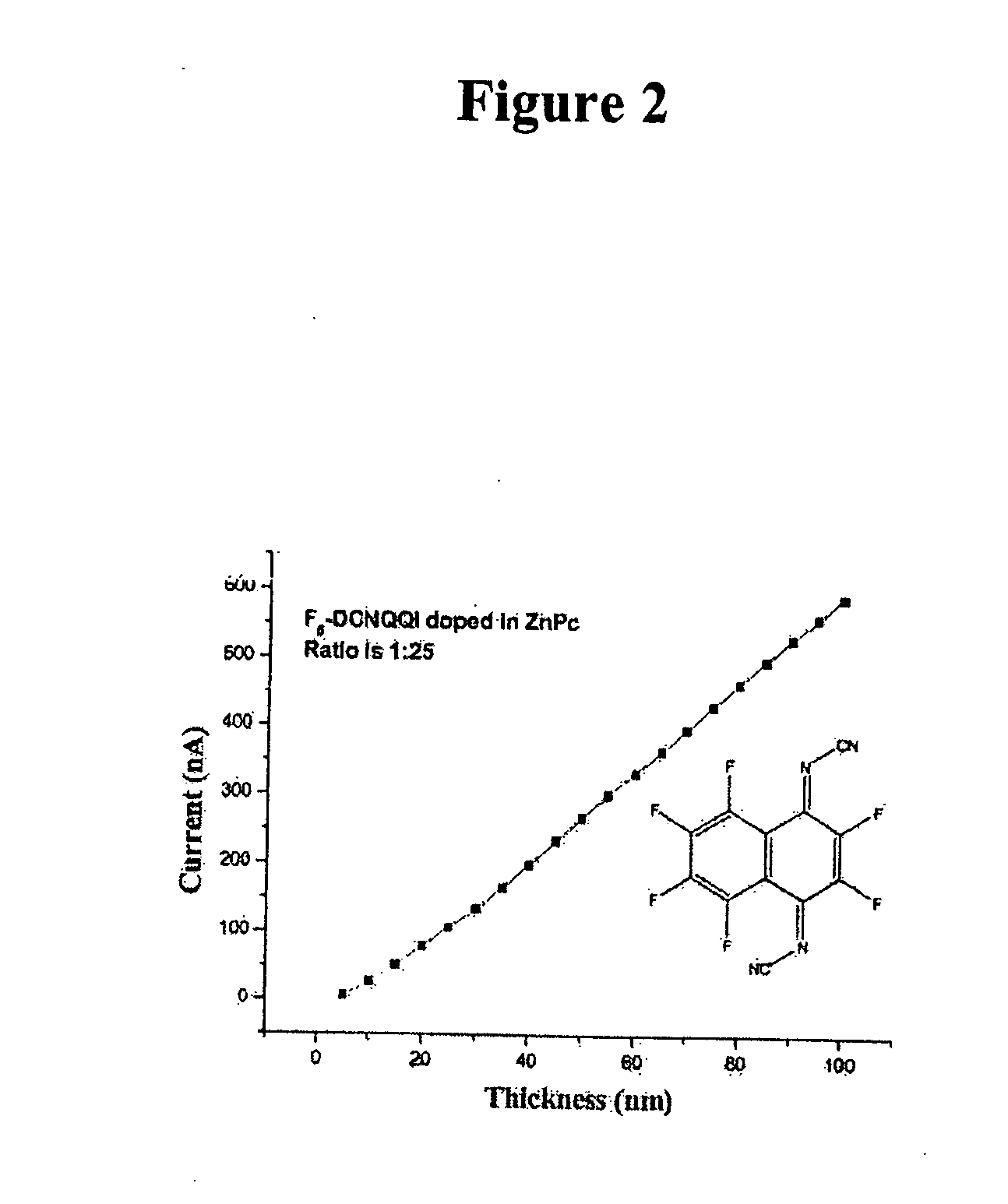
[0391] Doping of ZnPc with N,N′-dicyan-2,5-dichloro-1,4-quinonediimine (C12DCNQI) the evaporation temperature T(evap.) is 114° C. The ratio of the two compounds in the vapor-deposited layer is 1:50 in favor of the matrix. A conductivity of 1.0×10−2 s / cm was measured in the layer. Results are shown in FIG. 2 and Table 2 below.
TABLE 2Layer ThicknessCurrent(nm)(nA)542.6610179.415334.22048425635.53078635946401091.5451253501409.8
example 3
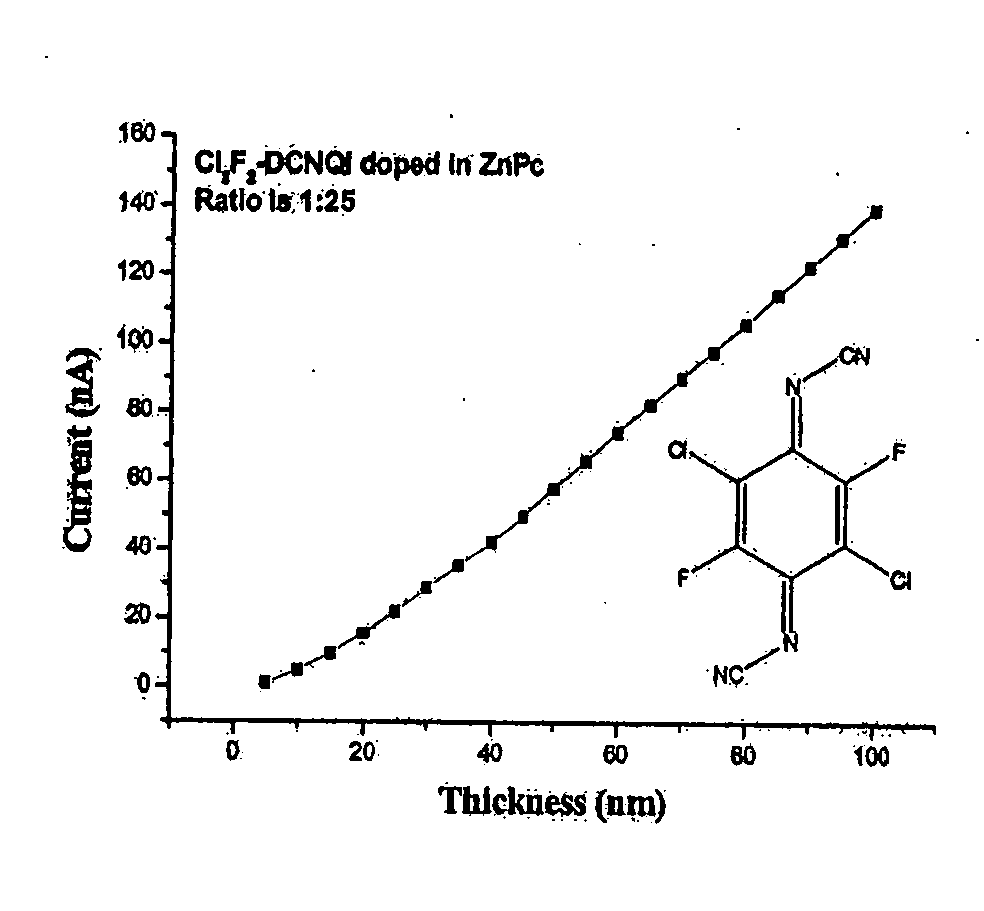
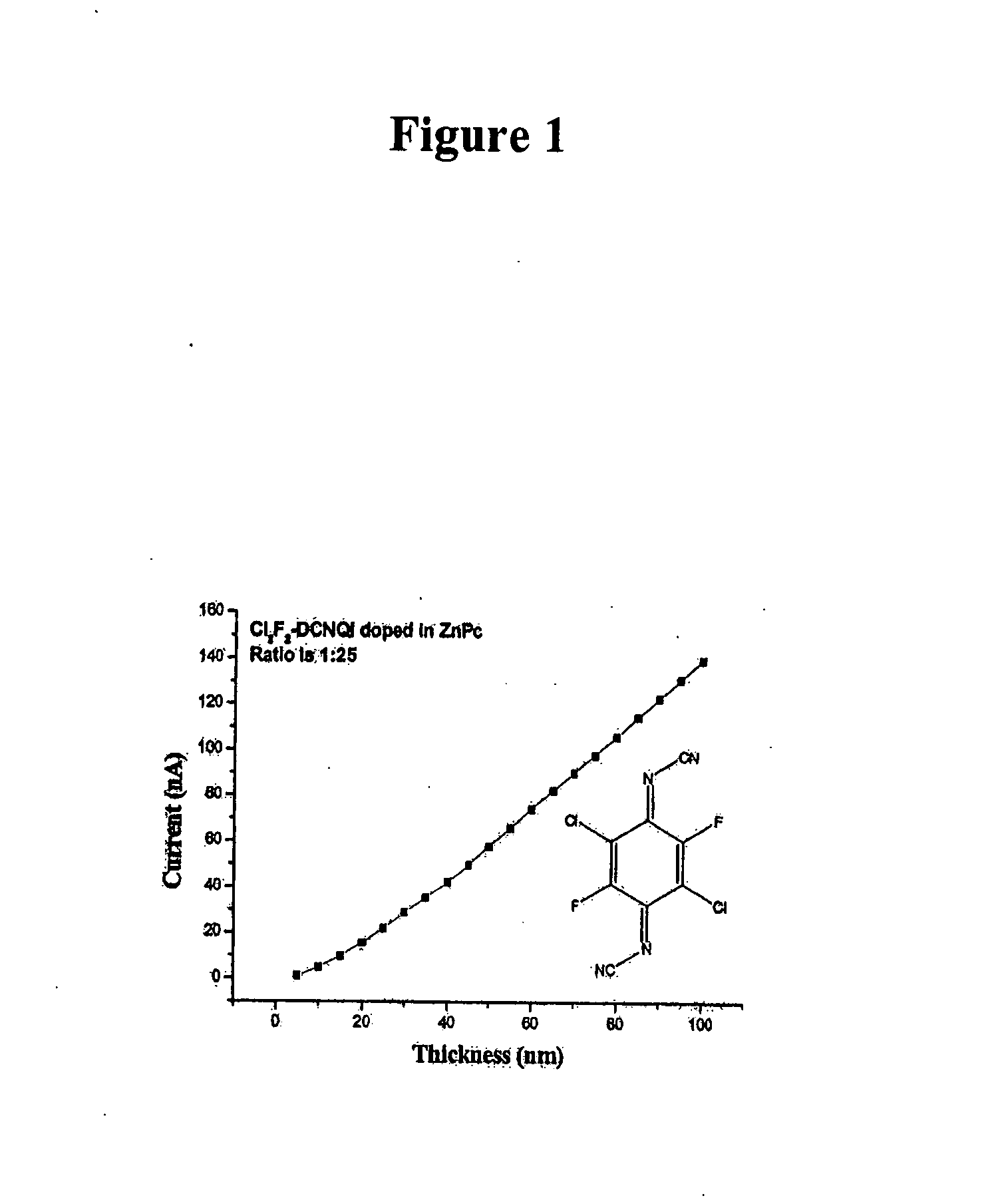
[0392] Doping of ZnPc with N,N′-dicyano-2,5-dichloro-3,6-difluoro-1,4-quinonediimine (C12F2DCNQI)
[0393] The evaporation temperature T(evap.) is 118° C. The layer was vapor-deposited under vacuum at the ratio of 1:25 (dopant matrix). A conductivity of 4.9×10−4 s / cm was measured there. Results are shown in FIG. 3 and Table 3 below.
TABLE 3LayerthicknessCurrent(nm)(nA)51.1648104.7852159.72112015.5822521.9853028.8663535.454042.2494549.7475057.865566.0126074.3356582.4497090.2517597.96880106.1485114.5890122.8495131.1100139.59
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com