Antifungal properties of various forms of sophorolipids
a technology of sophorolipids and antifungal properties, which is applied in the field of use, can solve problems such as not leading to well-defined pure compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
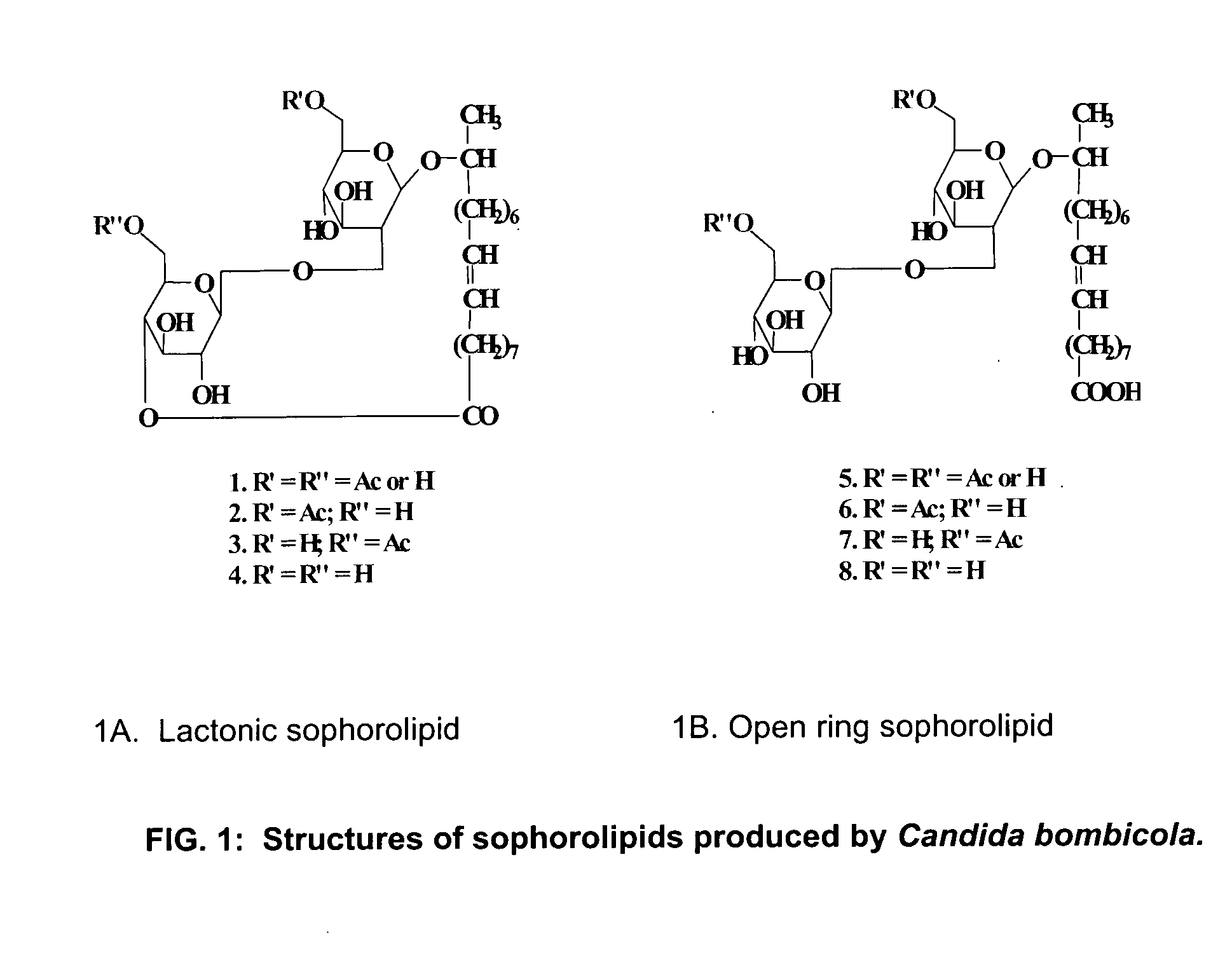
[0015] Sophorolipids were synthesized by fermentation of Candida bombicola. The fermentation media was composed of 100 g glucose, 10 g yeast extract, 1 g urea and 40 g oleic acid in 1000 ml of water. After 7 days of fermentation, sophorolipid was extracted thrice using ethyl acetate. The extracts were pooled and the solvent then was removed. The obtained product then was washed with hexane to remove the residual fatty acids. This was “natural” sophorolipid. The sophorolipid was dried in a vacuum desiccator.
2. Preparation of Lactonic Sophorolipid
[0016] Column chromatographic separations were performed over silica gel 70 (Aldrich Chemical Co.) to separate lactonic sophorolipid from the natural mixture. 50 g of silica gel was used to pack a glass column (5 cm×50 cm) in the eluent (CHCl3 / MeOH mixture). 200 ml of eluent was run through the column before the crude mixture (dissolved in a minimal volume of eluent) was loaded onto the top of the column matrix....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com