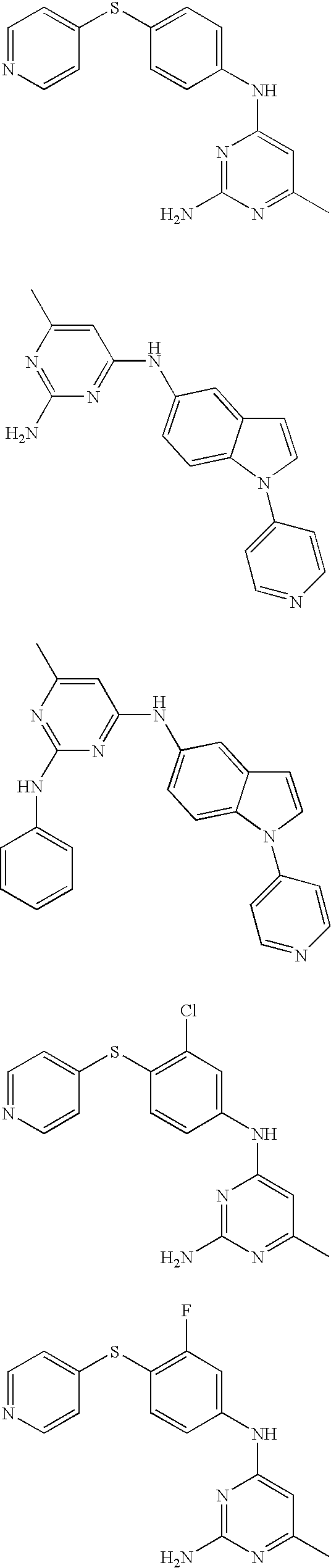
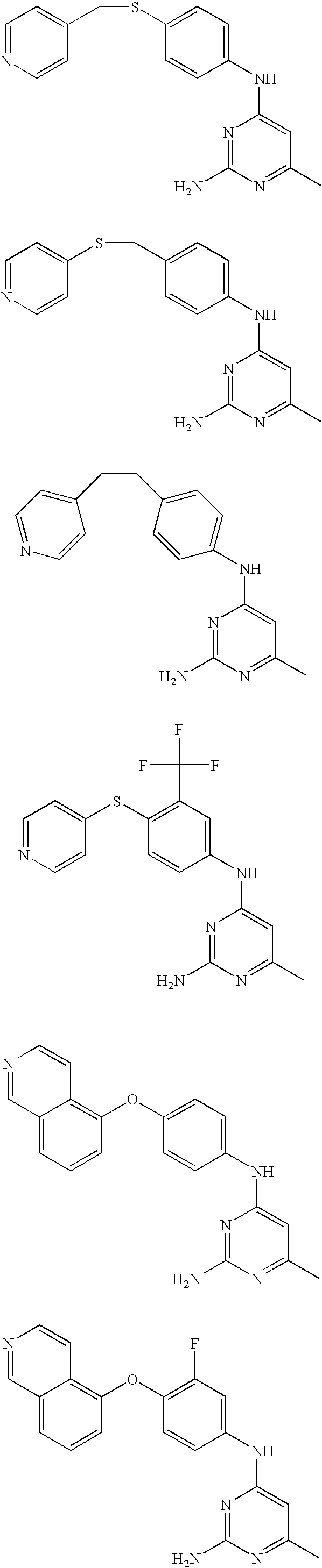
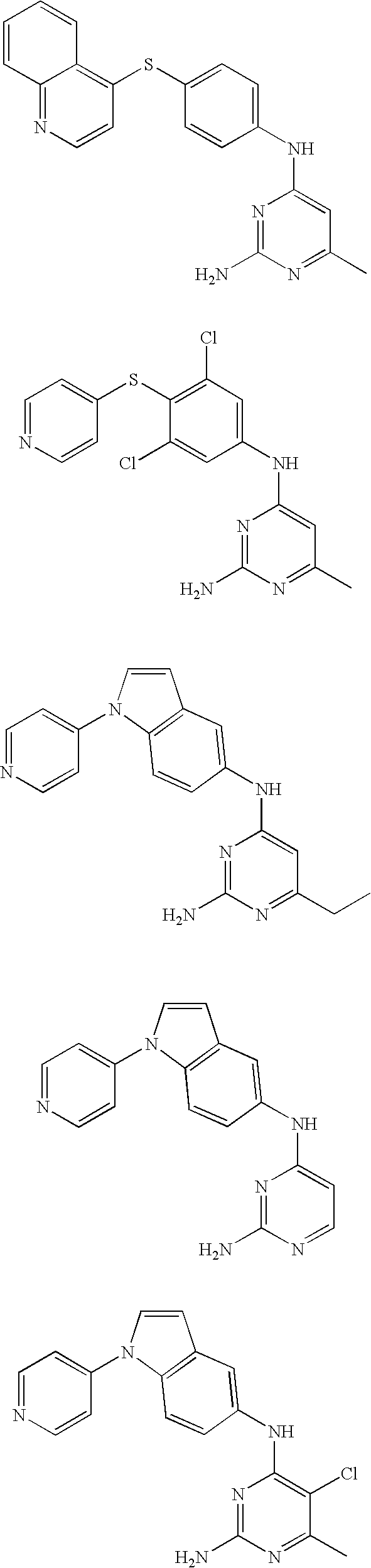
Rho-kinase inhibitors
a technology of rho-kinase and inhibitors, which is applied in the direction of biocide, drug composition, extracellular fluid disorder, etc., can solve the problem that diseases pose a serious unmet medical need
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of N-(2-amino-6-methyl-4-pyrimidinyl)-N-[3-fluoro-4-(4-pyridinylsulfanyl)phenyl]amine
[0081]
[0082] A suspension of 2-amino-4-chloro-6-methylpyrimidine (Intermediate A7, 0.2 g, 1.3 mmol), 3-fluoro-4-(4-pyridinylthio)aniline (Intermediate B7, 0.3 g, 1.3 mmol), and K2CO3 (0.2 g, 1.3 mmol) in o-xylene (1.3 mL) was heated to 100° C. in a 5 mL reaction-vial overnight. The reaction mixture was diluted with MeOH and coated on silica and purified by MPLC (Biotage) with 5-7% MeOH in CH2Cl2. It afforded 74 mg of product (18% yield). TLC (6% MeOH / 94% CH2Cl2) Rf 0.29; MS ES 328 [M+H]+; 1H-NMR (DMSO-d6) δ 2.12 (s, 3H), 5.92 (s, 1H), 6.38 (bs, 2H), 6.96 (d, J=5.1 Hz, 2H), 7.39-7.52 (m, 2H), 8.26 (d, J=11.9 Hz, 1H), 8.33 (d, J=4.8 Hz, 2H), 9.55 (bs, 1H).
example 2
Preparation of 6-ethyl-N4-[3-fluoro-4-(4-pyridinylsulfanyl)phenyl]-2,4-pyrimidinediamine
[0083]
[0084] 2-Amino-4-chloro-6-ethylpyrimidine (Intermediate A8, 55.1 mg, 0.25 mmol) and Intermediate B7(39.4 mg, 0.25 mmol) were suspended in 0.01 M aqueous HCl (500 μL). The mixture was refluxed for 6 h. The reaction was cooled to room temperature and the solvent was evaporated by vacuum. The residue was purified by reversed phase chromatography on a YMC Pack-pro C18 column (trademark) eluting with acetonitrile / H2O (10:90-90:10 gradient). The compound was further purified by preparative TLC eluting with CH2Cl2-MeOH (90: 10). Desired compound (2.9 mg, 0.0085 mmol; 34% yield); 1H NMR (Methanol-d4) 8.16 (dd, J=1.7, 4.7, 2H), 8.00-8.04 (m, 1H), 7.37 (m, 2H), 6.93 (dd, J=1.8, 4.9, 2H), 5.91 (s, 1), 2.39 (q, J=7.7, 2H), 1.13 (t, J=7.5, 3H); ES MS [M+H]+=342 ; TLC (CH2Cl2-MeOH, 90:10); Rf =0.48.
examples 3-26
[0085] Using the above procedures, the following examples of pyridines were synthesized and are summarized in Table 3.
TABLE 3IaIntermediateIntermediatePyrimidoneAmineEx. No.(A)(B)R1R2R4Analytical Data3A9B1HHamp = 245-247°C.; 1H NMR (Methanol-d4)8.79 (d, J = 5.7, 2H), 8.32 (s, 1H), 8.13 (d, J =5.9, 2H), 7.97 (d, J = 9.2, 1H), 7.88 (d, J =3.6, 1H), 7.71 (d, J = 7.3, 1H), 7.56 (d, J =9.1, 1H), 6.94 (d, J = 4.0, 1H), 6.33 (d, J =7.6, 1H); ES MS [M + H]+ = 303.4A1B1CH3CH2—Hamp = 230-233° C.; 1H NMR (DMSO-d6)12.43 (s, 1H), 10.51 (s, 1H), 8.79 (d, J = 6.3,2H), 8.22 (s, 1H), 7.87-8.23 (m, 5H), 7.46(d, J = 8.4, 1H), 6.85 (d, J = 3.3, 1H), 6.14 (s,1H), 2.51-2.61 (m, 2H), 1.19 (t, J = 7.5,3H); ES MS [M + H]+ 331.5A1B1CH3—Clamp = 238-241° C.; ES MS [M + H]+ = 351;TLC: Rf = 0.71 (CH2Cl2-MeOH, 95:5).6A1B1—(CH2)4—a1H NMR(DMSO-d6) 11.75 (s, 1H), 10.59 (s,1H), 8.78 (d, J = 5.4, 2H), 8.21 (s, 1H), 7.87-7.97 (m, 5H), 7.47 (d, J = 8.1, 1H), 6.85 (d,J = 3.4, 1H), 6.21 (s, 1H), 1.29 (s, 9H); ESMS [M + ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com