Intracorporeal occlusive device and method
a technology of intracorporeal occlusion and occlusion chamber, which is applied in the field of intracorporeal occlusion devices, can solve the problems of significant medical problems to the population, devastating consequences for patients, and high risk of procedures, and achieve the effect of preventing the egress of the space filling devi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
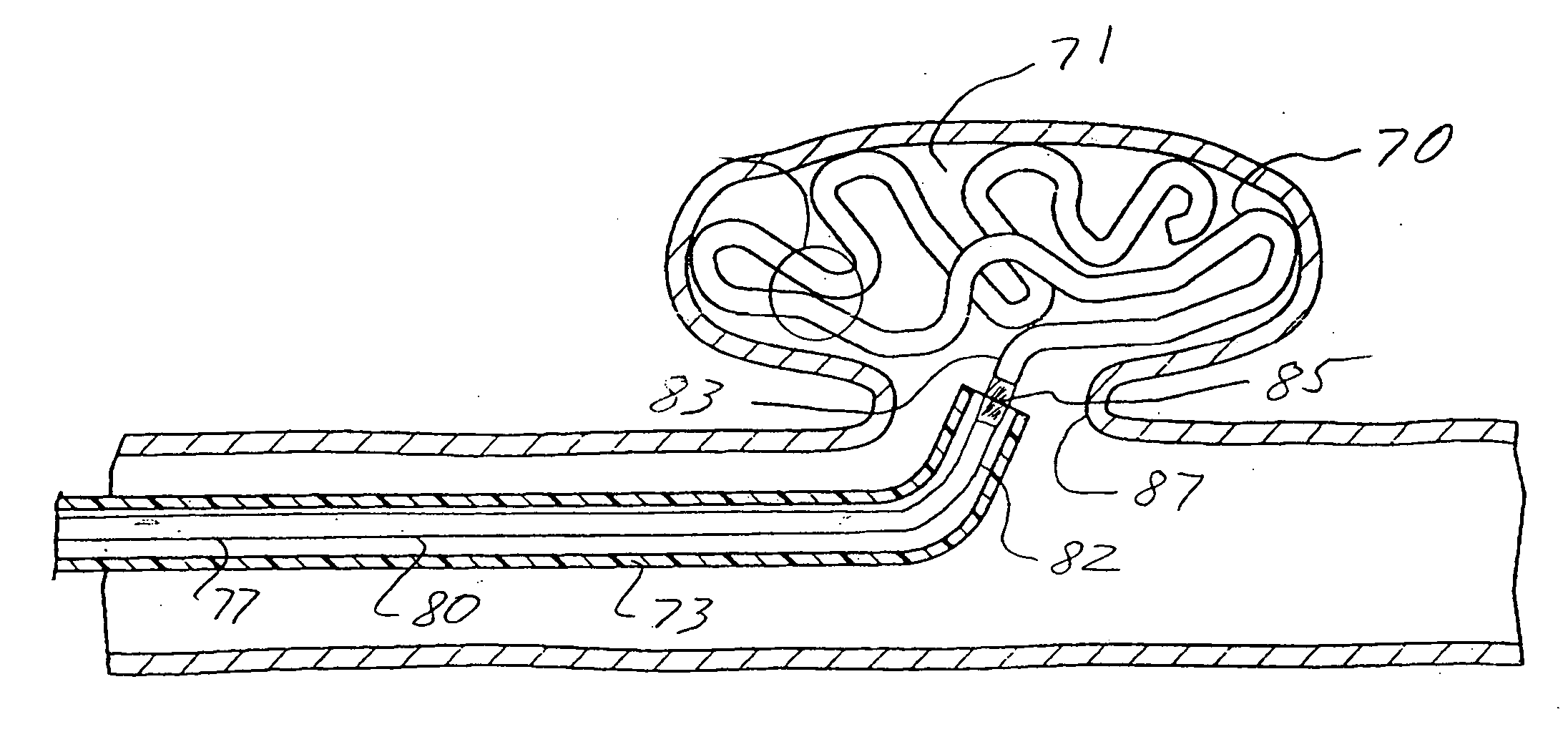
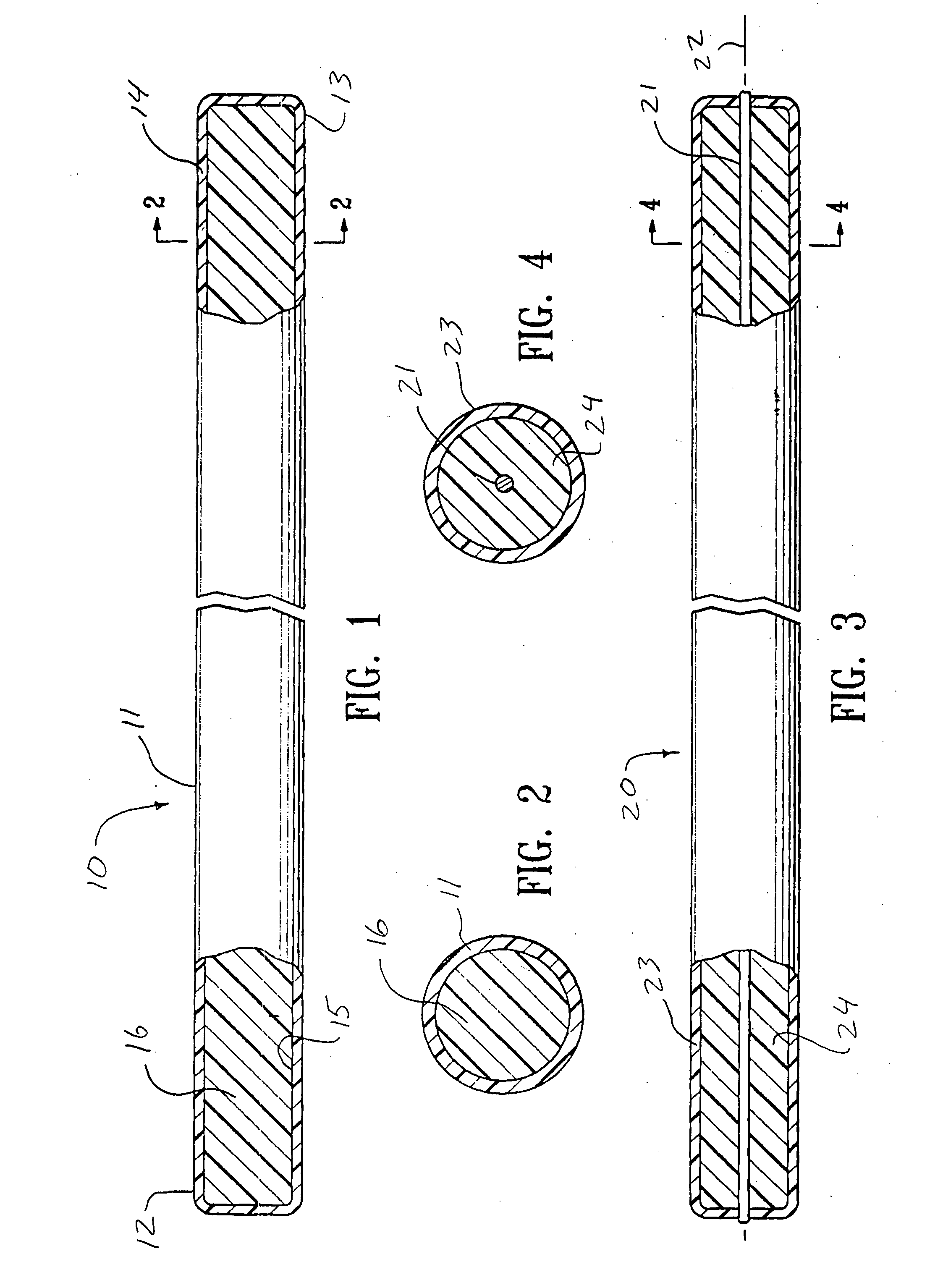
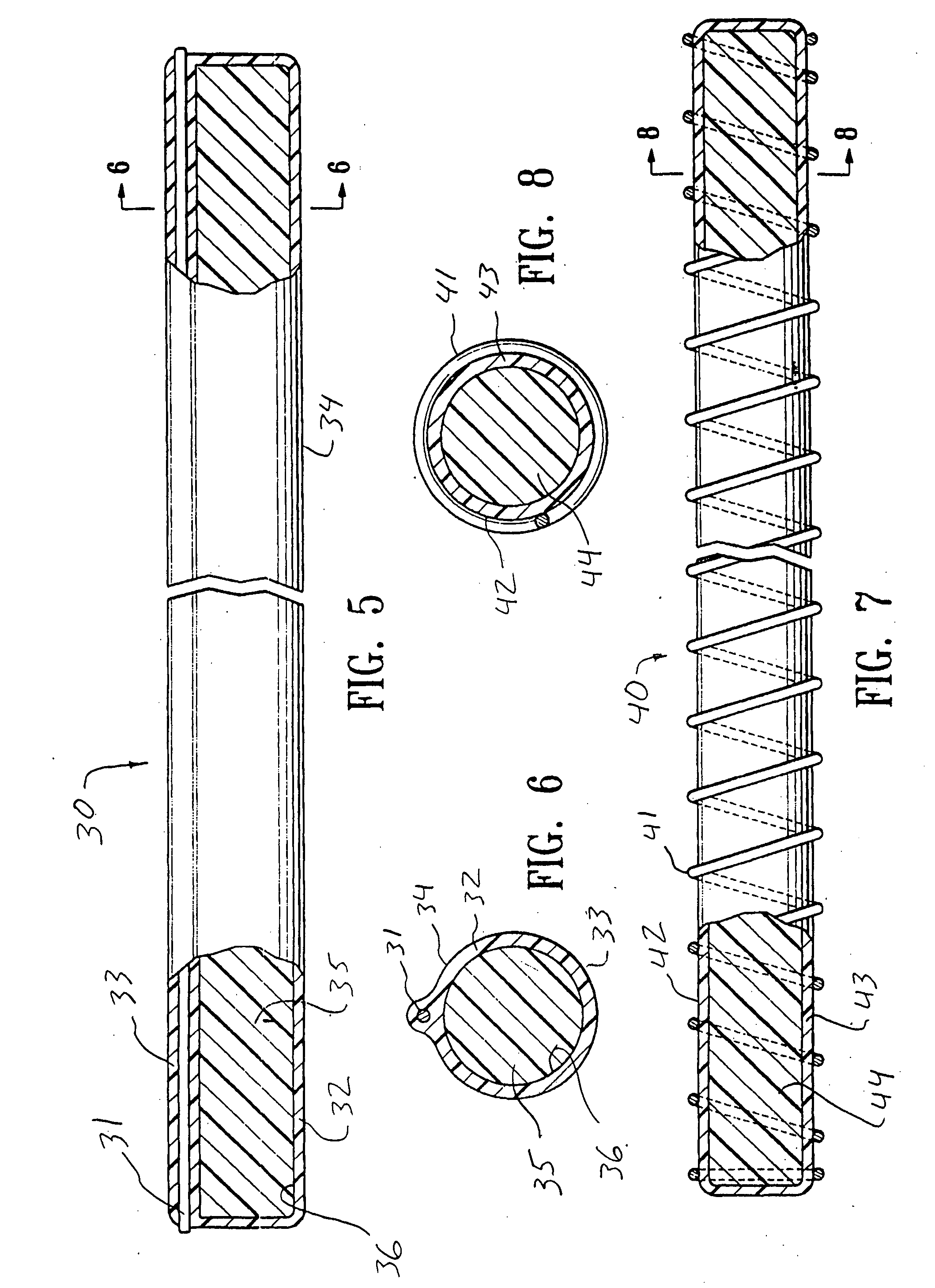
[0042]FIG. 1 illustrates an intracorporeal space filling device 10 having features of the invention. The intracorporeal space filling device 10 has an optional elongate tubular shell 11 with a first end 12 and a second end 13, the elongate shell being formed of a wall material 14. There is a lumen 15 disposed within the elongate tubular shell 11 which has transmutable material 16 disposed therein.
[0043] The elongate tubular shell 11 can be made from a variety of materials including metals and polymers. Suitable metals for the elongate tubular shell include stainless steel, NiTi, gold, platinum, tantalum, palladium, alloys thereof and the like. If a metal or other rigid material is used, methods such as forming slots or grooves in the wall material of such an elongate tubular shell may be used to achieve a desired longitudinal flexibility of the elongate tubular shell 11. Suitable polymers for the elongate tubular shell 11 can include polyurethane, polyethylene, nylon, polyimide, po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com