Methods and products related to the improved analysis of carbohydrates
a technology of improved analysis and carbohydrates, applied in the field of improved analysis of carbohydrates, can solve the problems of embryonic lethality, enormous multisystemic disorders,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
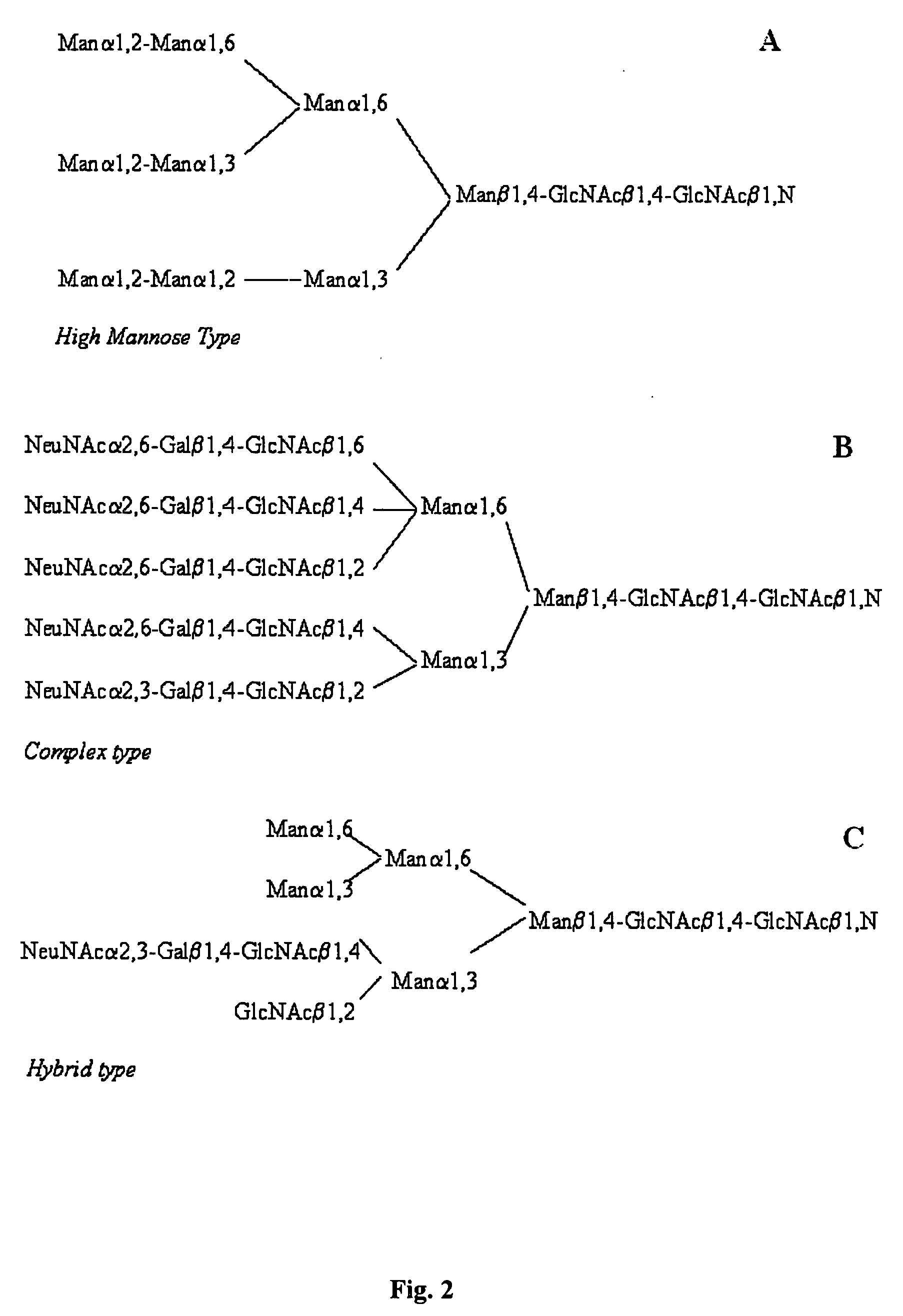
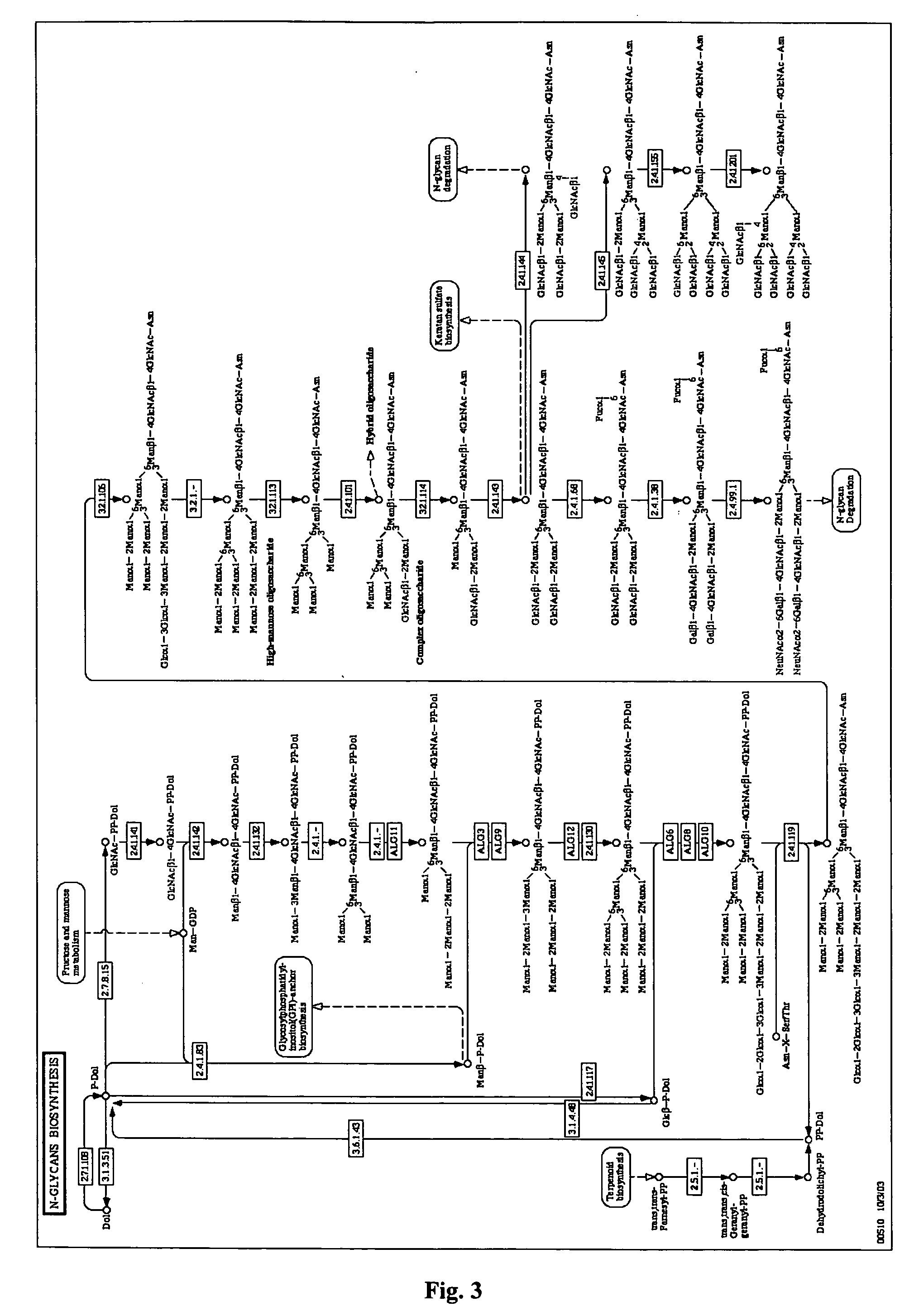
N-Glycan Analysis
Materials and Methods
PNGaseF Digest of N-Glycans from Protein Cores
[0153] Between 10 and 100 μg of protein was denatured for 10 minutes at 90° C. with 0.5% SDS and 1% β-mercaptoethanol. Since SDS (and other ionic detergents) inhibits enzyme activity, 1% NP-40 was added to counteract these effects. The enzyme reaction was performed overnight with 2 μl of PNGaseF at 37° C. in a 50 mM sodium phosphate buffer, pH 7.5.
Purification of Released N-Glycans
[0154] Proteins were precipitated with a 3× volume of 100% ethanol on ice for 1 hour. After centrifugation to remove the proteins, the supernatant containing the N-glycans was evaporated by vacuum (SpeedVac, TeleChem International, Inc., Sunnyvale, Calif.). Dried glycans were resuspended in 50 μl of water.
[0155] Samples were desalted using 1 ml ion exchange column of AG50W X-8 beads (Bio-Rad, Hercules, Calif.). The resin was charged with 150 mM acetic acid and washed with water. Glycan samples were loaded onto the ...
example 1 references
[0181] 1. Hirschberg, C. B., Snider, M. D. (1987) Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem 56, 63-87. [0182] 2. Bause, E. (1983) Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem J 209, 331-6. [0183] 3. Marshall, R. D. (1972) Glycoproteins. Annu Rev Biochem 41, 673-702. [0184] 4. Dwek, R. A. (1996) Glycobiology: Toward Understanding the Function of Sugars. Chem Rev 96, 683-720. [0185] 5. O'Connor, S. E., Imperiali, B. (1996) Modulation of protein structure and function by asparagine-linked glycosylation. Chem Biol 3, 803-12. [0186] 6. Crocker, P. R., Varki, A. (2001) Siglecs in the immune system. Immunology 103, 137-45. [0187] 7. Helenius, A., Aebi, M. (2001) Intracellular functions of N-linked glycans. Science 291, 2364-9. [0188] 8. Imperiali, B., O'Connor, S. E. (1999) Effect of N-linked glycosylation on glycopeptide and glycoprotein structure. Curr Opi...
example 2
Profiling of N-Glycans from Human Serum
Materials and Methods
Cleavage of N-Glycans from Serum Glycoproteins (Reduction / Carboxymethylation Method)
[0217] Human male normal serum samples were obtained from IMPATH (Franklin, Mass.) and Biomedical Resources (Hatboro, Pa.), and stored at −85° C. For each experiment, 50 μl of serum was used to harvest N-glycans. Serum samples were first diluted 1:4 with water, then DTT was added to a final concentration of 80 mM. After incubation for 30 minutes at 37° C., iodoacetic acid was added to a final concentration of 400 mM and incubated for 1 hour more at 37° C. The sample was dialyzed against 10 mM Tris acetate pH 8.3 overnight and concentrated to ˜200 μl in a spin column with a 3000 Da MWCO filter. To cleave the sugars from the protein, 5 μl (1,000 U) of PNGaseF (New England Biolabs, Beverly, Mass.) was added and allowed to react overnight at 37° C.
Purification of N-Glycans
[0218] After glycans were cleaved from the protein, the sample was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com