Handheld audiometric device and method for hearing testing
an audiometric device and hearing testing technology, applied in the field of hearing testing, can solve the problems of insufficient consideration of individual screening tests based on a single measurement, and insufficient consideration of the entire screening program. to achieve the effect of effective auditory screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The following detailed description illustrates the invention by way of example and not by way of limitation. The description enables one skilled in the art to make and use the invention, and describes several embodiments, adaptations, variations, alternatives, and uses of the invention, including what is presently believed to be the best mode of carrying out the invention.
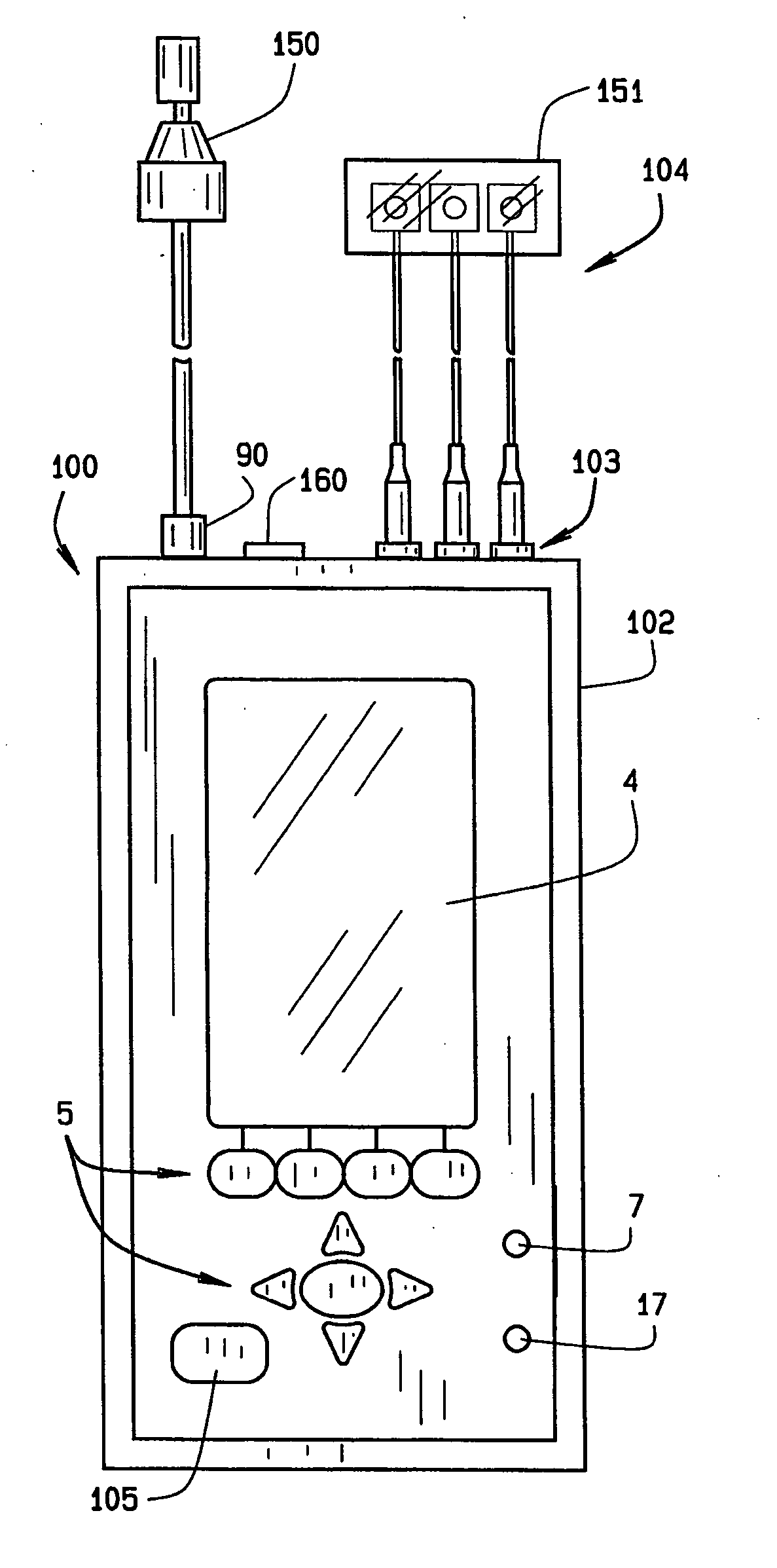
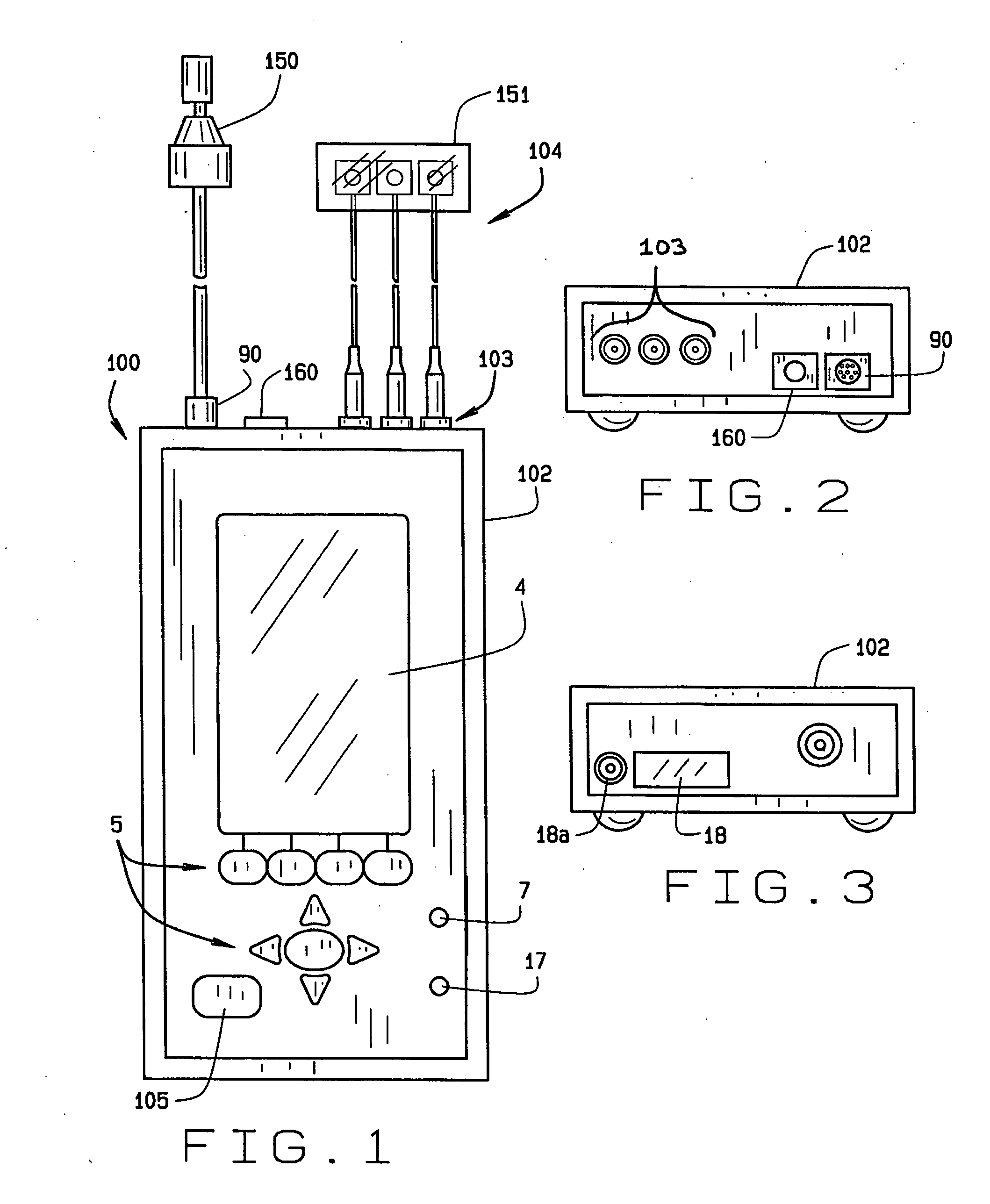
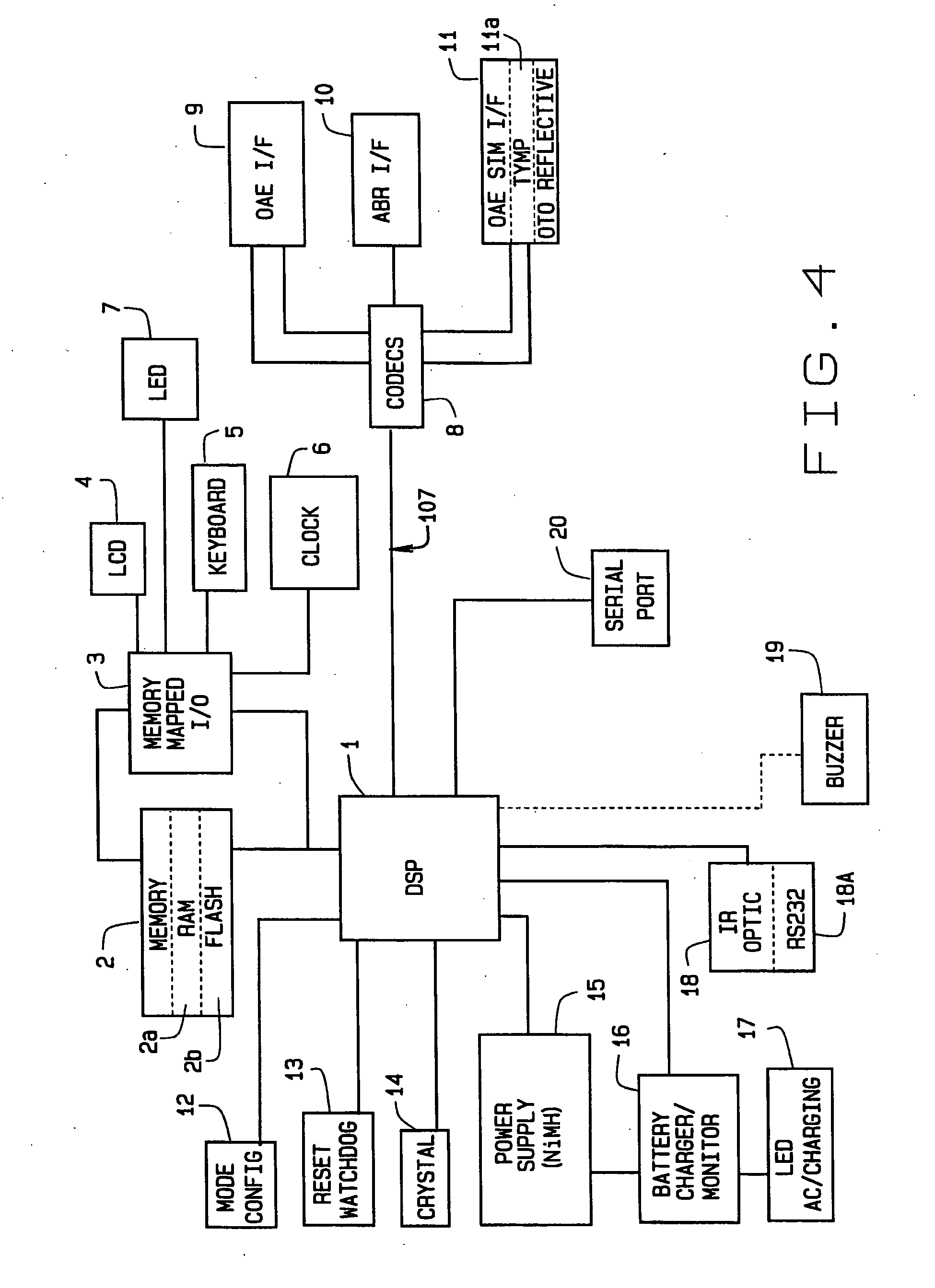
[0024] Referring now to FIGS. 1-3, reference numeral 100 illustrates one embodiment of the audio screening device of the present invention. The screening device 100 includes an enclosure 102, which in the preferred embodiment, and for purposes of illustration and not for limitation, measures 7 1 / 4″ long by 3¾ wide by 1½″ deep. It is important to note that the device 100 can be carried by the user without compromise, and truly represents a portable hand-held device having full functionality as described below. The device 100 includes a keyboard 5, an LCD display 4, an LED pass / refer indicator 7, and an LED A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com