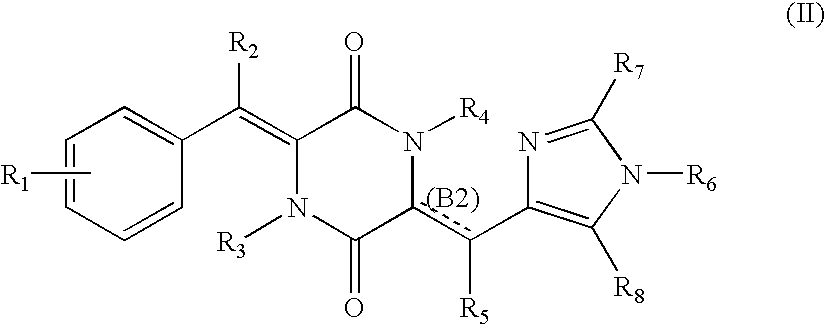
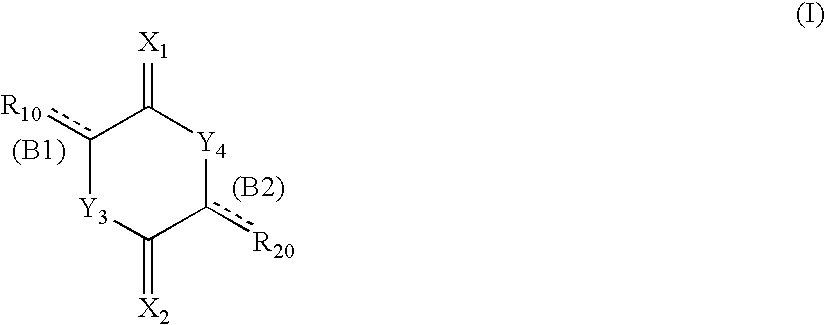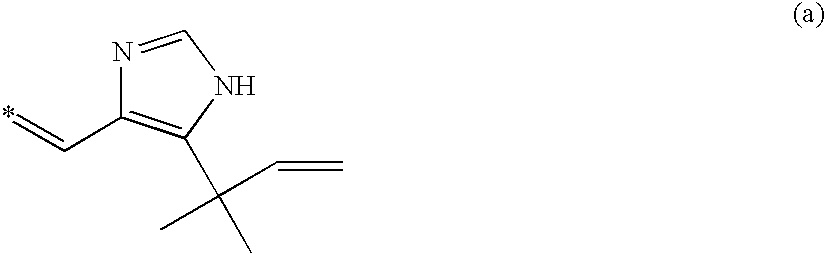Cell division inhibitor and a production method thereof
a cell division inhibitor and cell cycle technology, applied in the field of cell division inhibitors and antitumor agents, can solve problems such as immune disease development, cancer or immune disease, and achieve the effects of reducing the risk of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(1) Phenylahistin was Prepared as Follows.
[0102] Phenylahistin-producing bacterial cells (Aspergillus ustus NSC-F038, which was deposited with the National Institute of Bioscience and Human-Technology, Agency of Industrial Science and Technology (Higashi 1-1-3, Tsukuba-shi, Ibaragi-ken, Japan) under accession No. FERM P-15830 on Sep. 3, 1996), were inoculated onto five spots on a solid medium (20 ml per 9 cm dish) which contains 0.5% glucose, 2% glycerol, 0.2% yeast extract, 2% Pharmamedia (cottonseed cake), 0.25% sodium chloride and 1.5% agar (pH 6.5). The cells were then cultured at 26° C. for 7 days in the dark to obtain a spore suspension. The resulting spore suspension (0.1 ml) was inoculated onto each of 400 dishes containing 20 ml of the above solid medium, and then cultured at 26° C. for 8 days in the dark. The resulting culture was crushed using a mixer, and after addition of 8 L ethyl acetate, was allowed to stand for 2 days then extracted. The collected ethyl acetate la...
example 2
[0115] Dehydro-products of cyclophenylalanylhistidyl (CFH) were prepared from CFH through dehydrogenation as follows.
TABLE 4Reaction mixture compositionCFH0.5 mg / mlDimethyl sulfoxide10% (v / v)Sodium phosphate buffer (pH 8.0)9 mMCell-free extract from Example 10.435 units / ml
[0116] The reaction mixture (100 ml) shown in Table 4 was prepared and divided into five 20 ml Erlenmeyer flasks. The reaction was carried out in Reciprocal (160 strokes / min) at 50° C. for 24 hours. After 24 hours, the reaction mixture was centrifuged at 20,000×g for 15 min at 4° C. to obtain the supernatant. This supernatant was extracted with ethyl acetate, and then purified by HPLC (Waters 600 Controller, 486 Tunable Absorbance Detector, 616 Pump, Inertsil ODS-3 column φ20 mm×250 mm, 60% methanol as a solvent, flow rate of 10 ml / min, UV detection at 256 nm), thereby obtaining three dehydro-products at retention times of 3.9 min, 9.1 min and 11.6 min. Instrumental analysis indicates that the product eluted at 9...
example 3
[0121] A variety of dehydrodiketopiperazines were prepared from different diketopiperazines as substrates through dehydrogenation reactions using the enzyme of the present invention as follows.
TABLE 5Reaction mixture compositionDimethyl sulfoxide (DMSO)10% (v / v)Sodium phosphate buffer (pH 8.0)5.2 mMDichlorophenolindophenol (DCIP)80 μMPhenazine methosulfate (PMS)120 μMCell-free extract from Example 1q.s.Substrate0.5 mMTotal0.5 ml
[0122] The reaction mixture shown in Table 5 was used for the dehydrogenation reaction at 37° C. The reaction product was analyzed by HPLC and detected by UV absorbance at 256 nm. This method provided the following dehydro-products: ΔCAF, ΔCFF, ΔCFG, ΔCFH, ΔCFL, CΔFL, CFΔL, ΔCFS, ΔCFV, ΔCFW, ΔCLW, ΔCLY, ΔCVY, ΔCWW, ΔCWY, ΔCDWY (W residue is D-form), and ΔPLH.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com