Three-dimensional structural activity correlation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
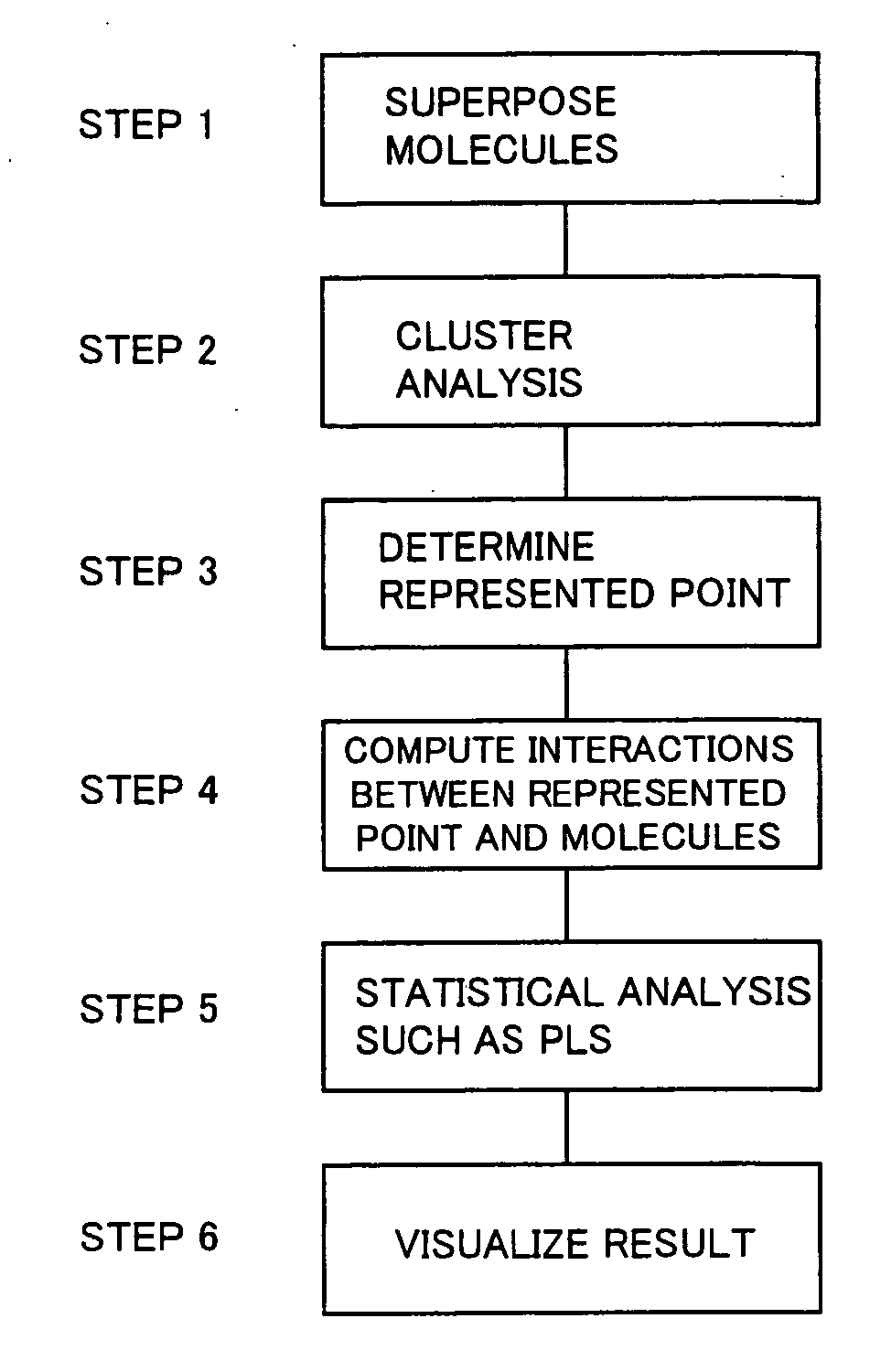
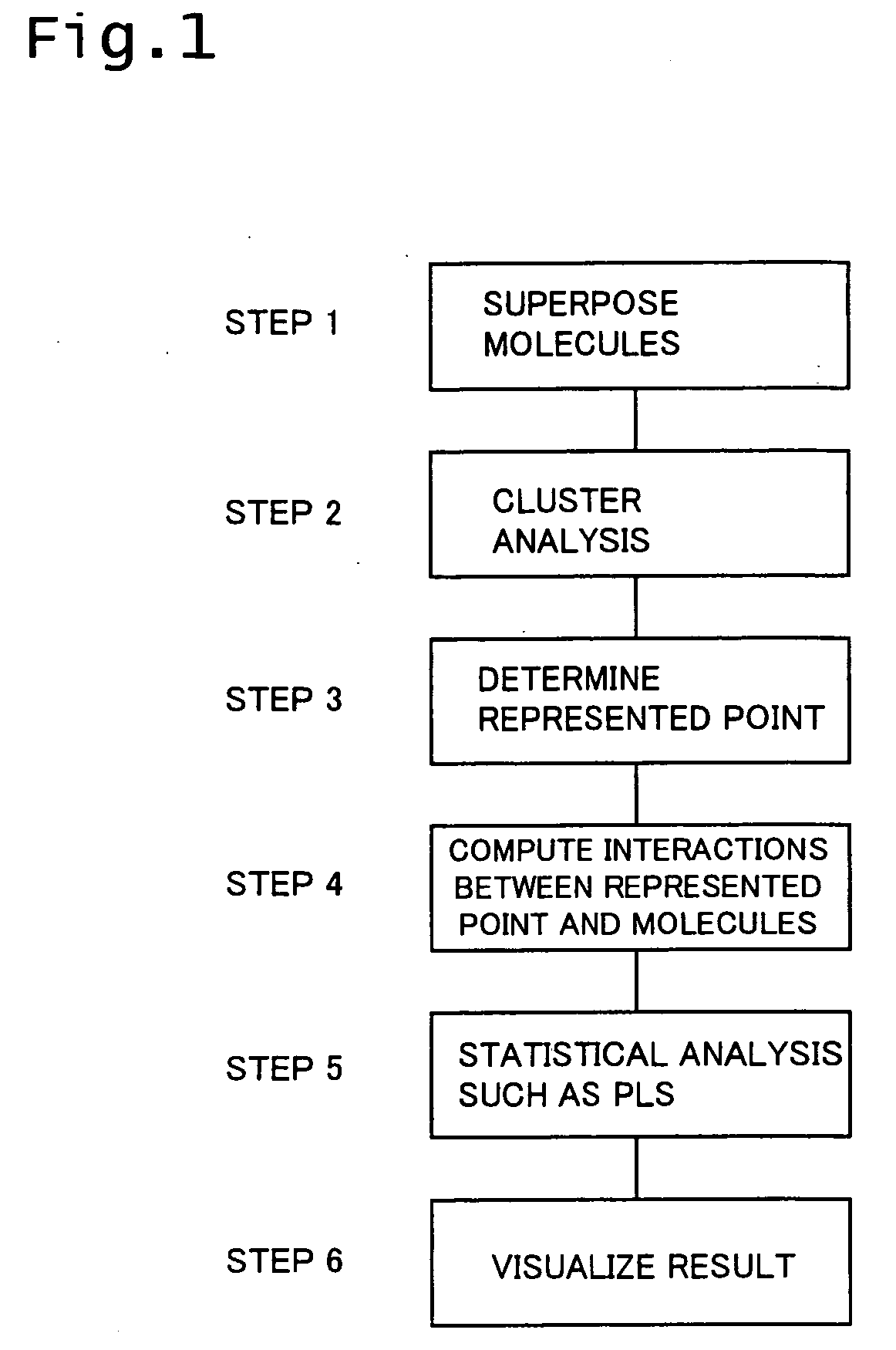
Method used
Image
Examples
example 1
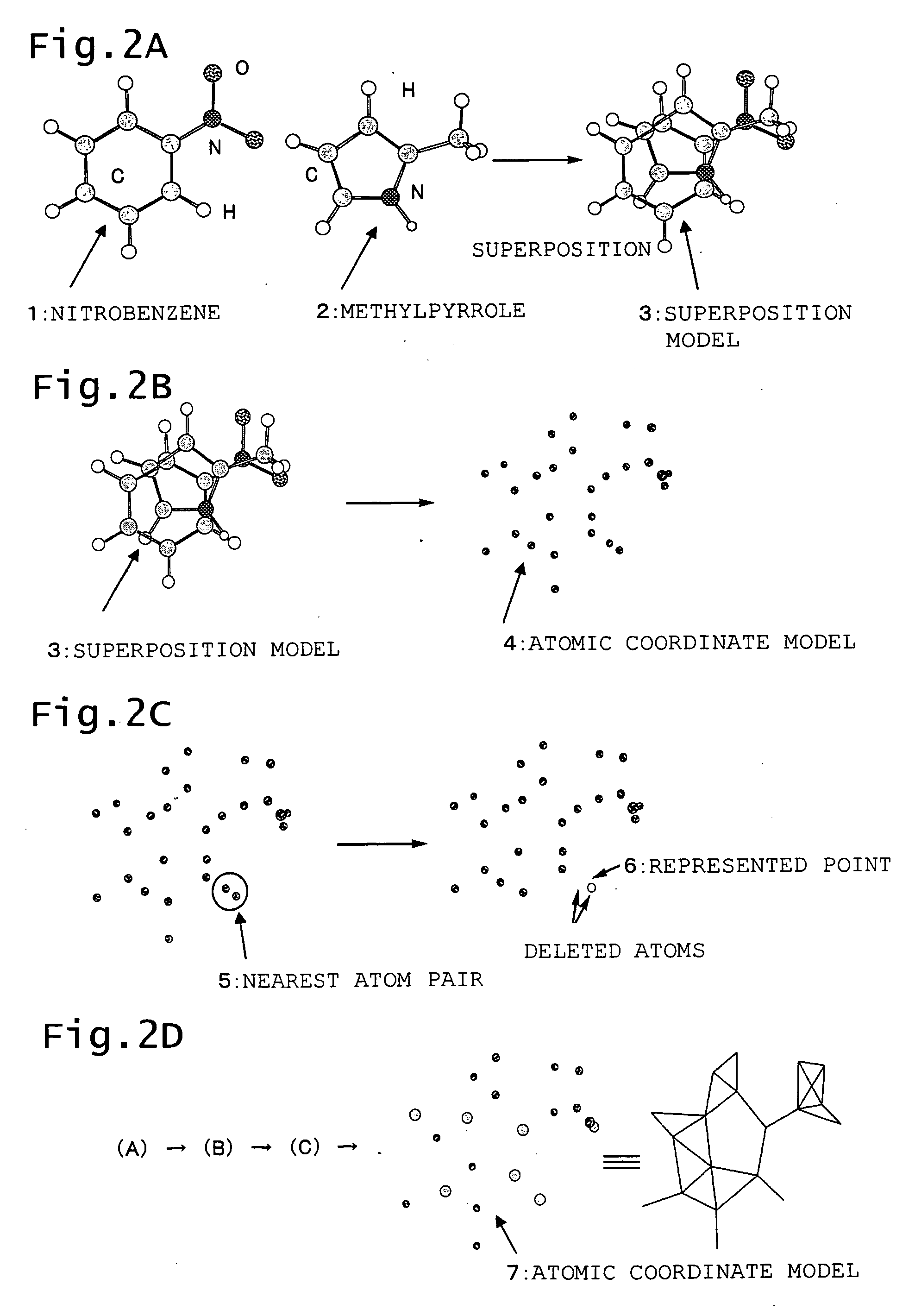
Generation of Represented Points Based on Atomic Coordinates of Superposed Molecules
1-A) Use of Rapid Molecular Superposition Evaluation Formula
[0138] After superposition, 0.3D QSAR analysis using molecular superposition method discussed in Kotani, T.; Higashiura, K. Rapid evaluation of molecular shape similarity index using pairwise calculation of the nearest atomic distances. J. Chem. Inf. Comput. Sci., 2002, 42, 58-63. was conducted. FIG. 9 shows the PLS analysis result. In FIG. 9, r2 is a multiple correlation coefficient, q2 is cross-validated r2, and 1−(n−1) (1−q2 / (n−c) is an evaluation function expressing the optimal number of components proposed by Tropsha et al. In this example, q2 has the maximum value when the number of components is 2, holding that this is a reliable model.
[0139]FIG. 10 is visualization of the computed result. In FIG. 10, the green portions are regions where the activity will be enhanced sterically, i.e., a sterically demanding substitutional group wi...
example 2
Generation of Represented Point with Addition of New Point at Position Representing Ring
[0143] A new point (pseudo-atom) wad added in a central section of a ring as a position which represents the ring, and similar computing was conducted. Addition of the pseudo-atom is expected to improve the accuracy of superposition and yield a more precise 3D QSAR result.
2-A) Use of Rapid Molecular Superposition Evaluation Formula
[0144] The same result as the 1-A result was obtained. This means that the rapid molecular superposition method which the inventors have developed is so accurate that it is not necessary to insert a pseudo-atom and permits superposition of molecules at a high accuracy.
2-B) Use of Seal-Type Evaluation Formula
[0145] After superposition with a pseudo-atom inserted at the center of a ring, 3D QSAR analysis was conducted using the SEAL-type evaluation method. FIG. 20 is a graph of r2, q2 and 1−(n−1) (1−q2) / (n−c). In this case, as q2 has the maximum value when the numb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com