Implantable heart assist system and method of applying same
a heart assist and implantable technology, applied in the field of extracardiac pumping system, can solve the problems of exertional dyspnea (difficult or laborious breathing), inability to maintain continuous flow, and nearly 250,000 patients per year to achieve the effect of revitalizing blood and low amount of energy inpu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
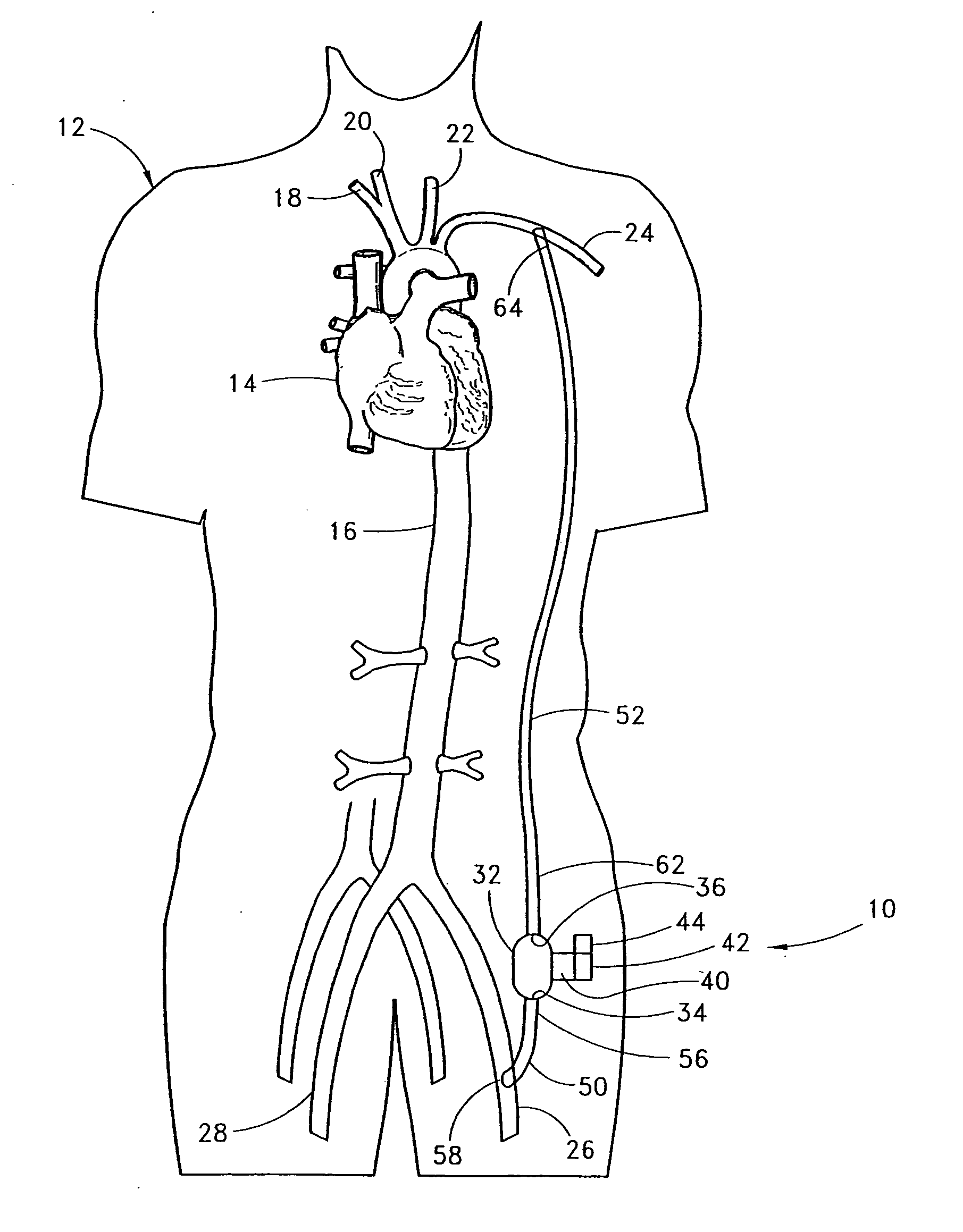
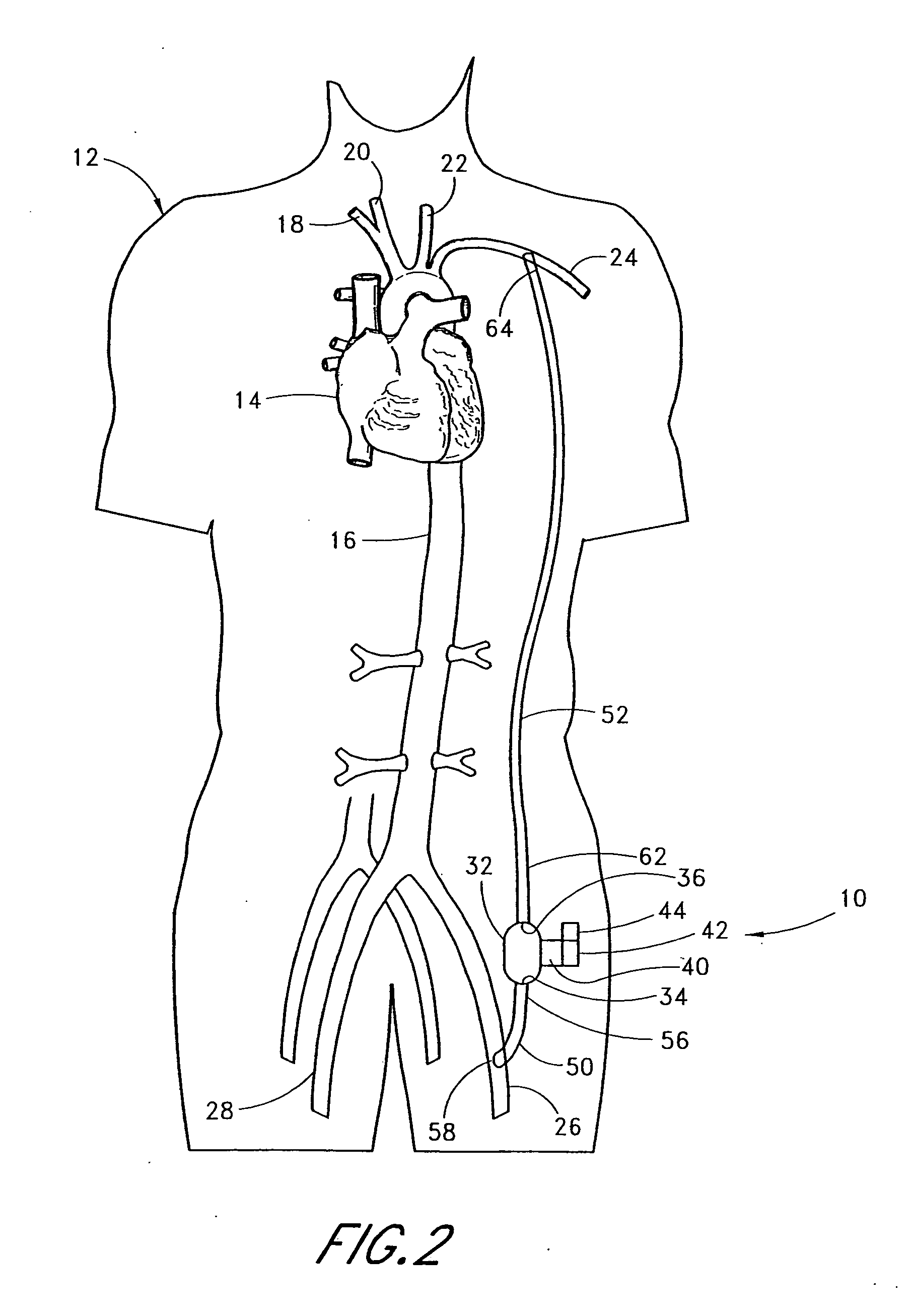
[0055] With reference to FIG. 2, the present invention 10 is shown applied to a patient 12 having an ailing heart 14 and an aorta 16, from which peripheral brachiocephalic blood vessels extend, including the right subclavian 18, the right carotid 20, the left carotid 22, and the left axillary 24. Extending from the descending aorta is another set of peripheral blood vessels, the left and right femoral arteries 26, 28.
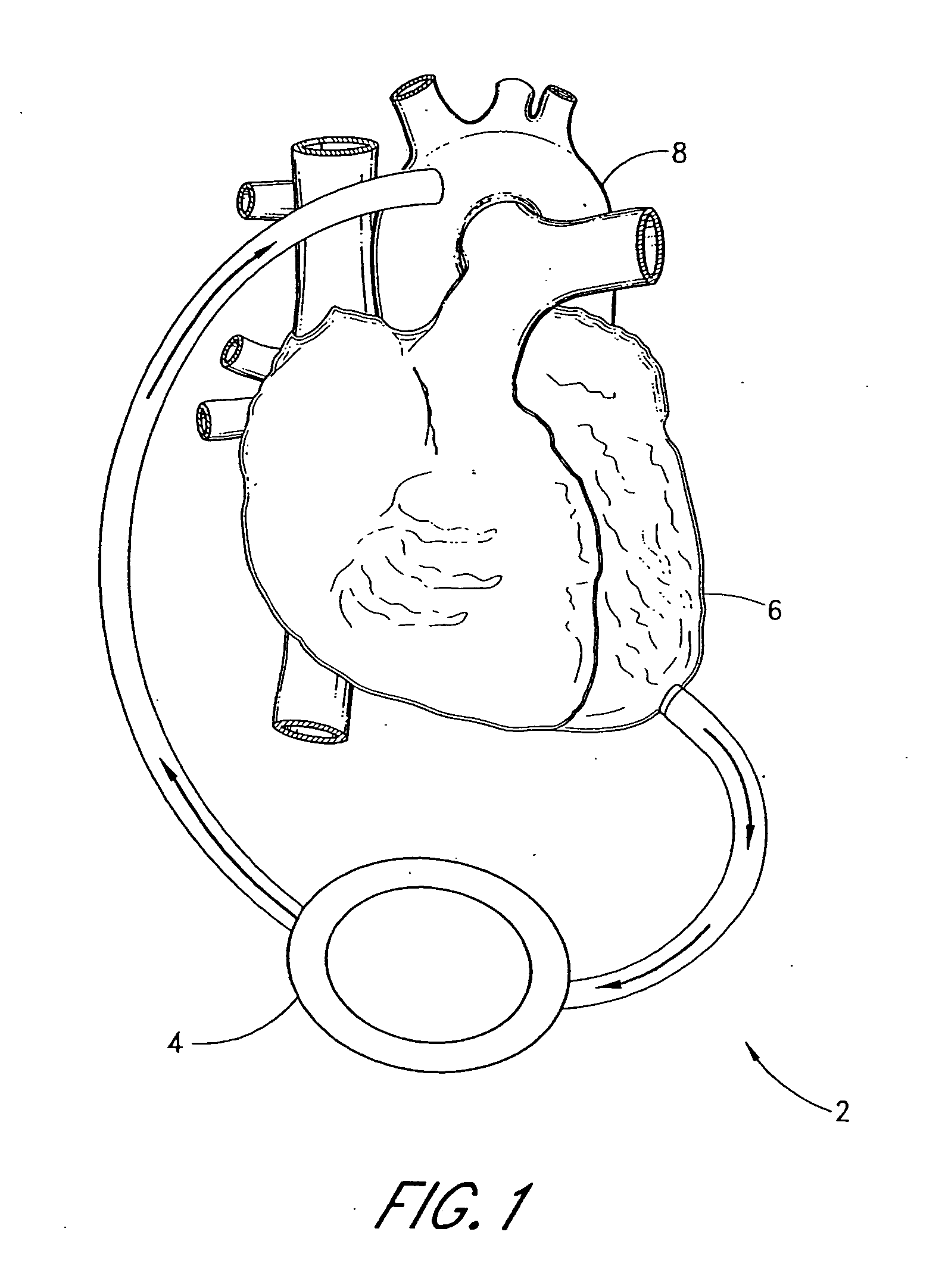
[0056] The first embodiment 10 comprises a pump 32, having an inlet 34 and an outlet 36 for connection of flexible conduits thereto. The pump 32 is preferably a rotary pump, either an axial type or a centrifugal type, although other types of pumps may be used, whether commercially-available or customized. In either case, the pump should be sufficiently small to be implanted subcutaneously and preferably extrathoracically, for example in the groin area of the patient, without the need for major invasive surgery. Because the present invention is an extracardiac system, no...
second embodiment
[0072] It is contemplated that, where an anastomosis connection is not desired, a special connector may be used to connect the conduits 50, 52 to the peripheral blood vessels. With reference to FIG. 3, the present invention is shown, wherein the inflow conduit 50 and outflow conduit 52 are connected to the peripheral blood vessels via first and second connectors 68, 70 each comprising three-opening fittings. In the preferred embodiment, the connectors 68, 70 comprise an intra-vascular, generally-tee-shaped fitting 72 having a proximal end 74, a distal end 76, and an angled divergence 78 permitting connection to the inflow and outflow conduits 50, 52 and the blood vessels. The proximal and distal ends 74, 76 of the fittings 72 permit connection to the blood vessel into which the fitting is positioned. The angle of divergence 78 of the fittings 72 may be 90 degrees or less in either direction from the axis of flow through the blood vessel, as optimally selected to generate the needed ...
third embodiment
[0078] A partial external application of the present invention is contemplated where a patient's heart failure is acute; i.e., is not expected to last long, or in the earlier stages of heart failure (where the patient is in New York Heart Association Classification (NYHAC) functional classes II or III). With reference to FIGS. 5 and 10, the present invention 110 is applied percutaneously to a patient 112 to connect two peripheral blood vessels wherein a pump 132 and its associated driving means and controls are employed extracorporeally. The pump 132 has an inflow conduit 150 and an outflow conduit 152 associated therewith for connection to two peripheral blood vessels. The inflow conduit 150 has a first end 156 and second end 158 wherein the second end is connected to a first peripheral blood vessel (e.g., femoral artery 126) by way of a cannula 180. The cannula 180 has a first end 182 sealably connected to the second end 158 of the inflow conduit 150. The cannula 180 also has a se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com